当前位置:
X-MOL 学术
›
J. Chem. Eng. Data
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Adsorption Equilibrium, Kinetics, and Thermodynamic Studies of Fluoride Adsorbed by Tetrametallic Oxide Adsorbent
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jced.8b00024 Sapna Raghav 1 , Dinesh Kumar 2
Journal of Chemical & Engineering Data ( IF 2.6 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1021/acs.jced.8b00024 Sapna Raghav 1 , Dinesh Kumar 2
Affiliation
|
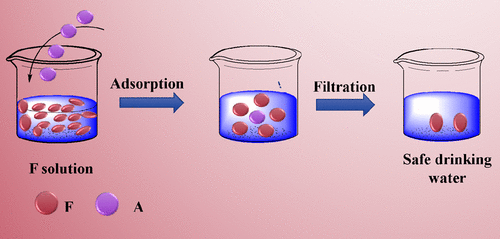
This study investigated the performance of fluoride adsorption onto a specific tetrametallic oxide adsorbent Fe–Al–Ce-Ni (FACN) and the effect of temperature on adsorption performance. The adsorption performance was determined by adsorption equilibrium, kinetics, and thermodynamic parameters. The adsorption, kinetic, and thermodynamic parameters were compared alternatively. The fluoride adsorption capacity was obtained from four different adsorption isotherm models, namely, Langmuir, Freundlich, Temkin, and Dubinin–Radushkevich (D–R), and Freundlich was found to best fit model. Fluoride removal rate using adsorption (0.27 min–1) was obtained faster than reactive adsorption (0.04 min–1). Several thermodynamic parameters such as enthalpy, Gibbs free energy, entropy (ΔS > 0), and adsorption activation energy were calculated which demonstrated the feasibility and spontaneity (ΔG < 0) and exothermic nature of (ΔH < 0) the fluoride adsorption process. The adsorption process was controlled by a physical mechanism, and the maximum adsorption capacity was found to be 250 mg/g. To our knowledge, this is the first report on the synthesis of tetrametallic oxide adsorbent for fluoride adsorption, and the feasibility of the adsorption process was ratified by three van’t Hoff plots.
中文翻译:

四金属氧化物吸附剂吸附氟化物的吸附平衡,动力学和热力学研究
这项研究调查了氟化物在特定的四金属氧化物吸附剂Fe–Al–Ce-Ni(FACN)上的吸附性能以及温度对吸附性能的影响。吸附性能由吸附平衡,动力学和热力学参数确定。交替比较吸附,动力学和热力学参数。氟化物的吸附容量是从四种不同的吸附等温线模型获得的,即Langmuir,Freundlich,Temkin和Dubinin-Radushkevich(D-R),发现Freundlich是最佳拟合模型。使用吸附(0.27 min –1)获得的氟化物去除速率要比反应性吸附(0.04 min –1)更快。几个热力学参数,例如焓,吉布斯自由能,熵(ΔS> 0),并计算了吸附活化能,证明了氟化物吸附过程的可行性和自发性(ΔG <0)和放热性质(ΔH <0)。通过物理机制控制吸附过程,发现最大吸附容量为250mg / g。据我们所知,这是有关用于氟化物吸附的四金属氧化物吸附剂合成的第一份报告,并且通过三个van't Hoff曲线证明了吸附过程的可行性。
更新日期:2018-03-14
中文翻译:

四金属氧化物吸附剂吸附氟化物的吸附平衡,动力学和热力学研究
这项研究调查了氟化物在特定的四金属氧化物吸附剂Fe–Al–Ce-Ni(FACN)上的吸附性能以及温度对吸附性能的影响。吸附性能由吸附平衡,动力学和热力学参数确定。交替比较吸附,动力学和热力学参数。氟化物的吸附容量是从四种不同的吸附等温线模型获得的,即Langmuir,Freundlich,Temkin和Dubinin-Radushkevich(D-R),发现Freundlich是最佳拟合模型。使用吸附(0.27 min –1)获得的氟化物去除速率要比反应性吸附(0.04 min –1)更快。几个热力学参数,例如焓,吉布斯自由能,熵(ΔS> 0),并计算了吸附活化能,证明了氟化物吸附过程的可行性和自发性(ΔG <0)和放热性质(ΔH <0)。通过物理机制控制吸附过程,发现最大吸附容量为250mg / g。据我们所知,这是有关用于氟化物吸附的四金属氧化物吸附剂合成的第一份报告,并且通过三个van't Hoff曲线证明了吸附过程的可行性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号