当前位置:
X-MOL 学术
›
Environmental Science Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Sulfidation mechanisms of Fe(iii)-(oxyhydr)oxide nanoparticles: a spectroscopic study
Environmental Science Nano Pub Date : 2018-03-13 , DOI: 10.1039/c7en01109a Naresh Kumar 1, 2, 3, 4, 5 , Juan Lezama Pacheco 2, 3, 4, 5, 6 , Vincent Noël 5, 7, 8, 9 , Gabrielle Dublet 1, 2, 3, 4, 5 , Gordon E. Brown 1, 2, 3, 4, 5
中文翻译:

Fe(iii)-(羟基)氧化物纳米粒子的硫化机制:光谱研究
更新日期:2018-03-13
Environmental Science Nano Pub Date : 2018-03-13 , DOI: 10.1039/c7en01109a Naresh Kumar 1, 2, 3, 4, 5 , Juan Lezama Pacheco 2, 3, 4, 5, 6 , Vincent Noël 5, 7, 8, 9 , Gabrielle Dublet 1, 2, 3, 4, 5 , Gordon E. Brown 1, 2, 3, 4, 5
Affiliation
|
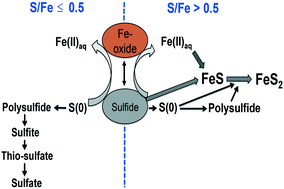
We used synchrotron-based X-ray absorption spectroscopy, transmission electron microscopy, and wet chemical analyses to study the sulfidation mechanism(s) and sulfur oxidation products from the reaction of ferrihydrite, goethite, and hematite nanoparticles with dissolved sulfide at different S/Fe molar ratios under anaerobic condition.
中文翻译:

Fe(iii)-(羟基)氧化物纳米粒子的硫化机制:光谱研究
我们使用基于同步加速器的 X 射线吸收光谱、透射电子显微镜和湿化学分析来研究水铁矿、针铁矿和赤铁矿纳米颗粒与不同 S/Fe 下溶解的硫化物反应的硫化机理和硫氧化产物。厌氧条件下的摩尔比。



























 京公网安备 11010802027423号
京公网安备 11010802027423号