当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Catalytic C−H Trifluoromethoxylation of Arenes and Heteroarenes
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201800598 Weijia Zheng 1 , Cristian A Morales-Rivera 2 , Johnny W Lee 1 , Peng Liu 2 , Ming-Yu Ngai 1
Angewandte Chemie International Edition ( IF 16.6 ) Pub Date : 2018-03-13 , DOI: 10.1002/anie.201800598 Weijia Zheng 1 , Cristian A Morales-Rivera 2 , Johnny W Lee 1 , Peng Liu 2 , Ming-Yu Ngai 1
Affiliation
|
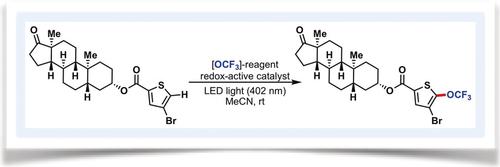
The intermolecular C−H trifluoromethoxylation of arenes remains a long‐standing and unsolved problem in organic synthesis. Herein, we report the first catalytic protocol employing a novel trifluoromethoxylating reagent and redox‐active catalysts for the direct (hetero)aryl C−H trifluoromethoxylation. Our approach is operationally simple, proceeds at room temperature, uses easy‐to‐handle reagents, requires only 0.03 mol % of redox‐active catalysts, does not need specialized reaction apparatus, and tolerates a wide variety of functional groups and complex structures such as sugars and natural product derivatives. Importantly, both ground‐state and photoexcited redox‐active catalysts are effective. Detailed computational and experimental studies suggest a unique reaction pathway where photoexcitation of the trifluoromethoxylating reagent releases the OCF3 radical that is trapped by (hetero)arenes. The resulting cyclohexadienyl radicals are oxidized by redox‐active catalysts and deprotonated to form the desired products of trifluoromethoxylation.
中文翻译:

芳烃和杂芳烃的催化 C−H 三氟甲氧基化
芳烃的分子间CH-C三氟甲氧基化仍然是有机合成中长期存在且未解决的问题。在此,我们报告了第一个采用新型三氟甲氧基化试剂和氧化还原活性催化剂进行直接(杂)芳基 C−H 三氟甲氧基化的催化方案。我们的方法操作简单,在室温下进行,使用易于处理的试剂,仅需要 0.03 mol% 的氧化还原活性催化剂,不需要专门的反应设备,并且可以耐受多种官能团和复杂结构,例如糖和天然产物衍生物。重要的是,基态和光激发氧化还原活性催化剂都是有效的。详细的计算和实验研究表明了一种独特的反应途径,其中三氟甲氧基化试剂的光激发释放被(杂)芳烃捕获的OCF 3自由基。所得环己二烯基自由基被氧化还原活性催化剂氧化并去质子化,形成所需的三氟甲氧基化产物。
更新日期:2018-03-13
中文翻译:

芳烃和杂芳烃的催化 C−H 三氟甲氧基化
芳烃的分子间CH-C三氟甲氧基化仍然是有机合成中长期存在且未解决的问题。在此,我们报告了第一个采用新型三氟甲氧基化试剂和氧化还原活性催化剂进行直接(杂)芳基 C−H 三氟甲氧基化的催化方案。我们的方法操作简单,在室温下进行,使用易于处理的试剂,仅需要 0.03 mol% 的氧化还原活性催化剂,不需要专门的反应设备,并且可以耐受多种官能团和复杂结构,例如糖和天然产物衍生物。重要的是,基态和光激发氧化还原活性催化剂都是有效的。详细的计算和实验研究表明了一种独特的反应途径,其中三氟甲氧基化试剂的光激发释放被(杂)芳烃捕获的OCF 3自由基。所得环己二烯基自由基被氧化还原活性催化剂氧化并去质子化,形成所需的三氟甲氧基化产物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号