当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Copper nitrate: a privileged reagent for organic synthesis
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8ob00332g Mingchun Gao 1, 2, 3, 4, 5 , Rongxuan Ye 1, 2, 3, 4, 5 , Weijia Shen 1, 2, 3, 4, 5 , Bin Xu 1, 2, 3, 4, 5
Organic & Biomolecular Chemistry ( IF 3.2 ) Pub Date : 2018-03-14 00:00:00 , DOI: 10.1039/c8ob00332g Mingchun Gao 1, 2, 3, 4, 5 , Rongxuan Ye 1, 2, 3, 4, 5 , Weijia Shen 1, 2, 3, 4, 5 , Bin Xu 1, 2, 3, 4, 5
Affiliation
|
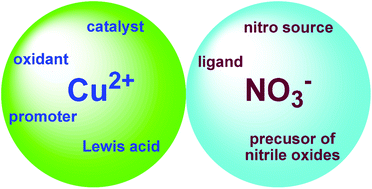
Copper has been explored as an ideal candidate for replacing noble metals in organic synthesis, especially for practical large scale preparation. Recent decades have witnessed the renaissance and improvement of copper-catalyzed and copper-mediated organic reactions. Copper nitrate is a common inorganic copper salt which has been proved to be a ubiquitous reactant in organic synthesis due to its commercial availability, stability, inexpensiveness and environmentally benign nature. Copper nitrate could be used as a nitration reagent, oxidant, catalyst or promoter, and Lewis acid as well. Remarkably, great attention has been devoted to the efficient transformation of copper nitrate into functionalized or complicated compounds through various reaction types including cyclization, C–H activation, difunctionalization, nitration, rearrangement and asymmetric synthesis with chiral ligands. Further modification of copper nitrate, such as solid-supported copper nitrate or copper nitrate complexes, extends its applications in organic synthesis. The present review highlights recent advances of copper nitrate in organic synthesis, along with the mechanisms.
中文翻译:

硝酸铜:有机合成的特权试剂
铜已被认为是替代有机合成中贵金属的理想候选者,特别是对于大规模实践而言。最近几十年见证了铜催化的和铜介导的有机反应的复兴和改善。硝酸铜是一种常见的无机铜盐,由于其商业上的可获得性,稳定性,廉价和对环境有益的性质,已被证明是有机合成中普遍存在的反应物。硝酸铜可用作硝化试剂,氧化剂,催化剂或促进剂,以及路易斯酸。值得注意的是,通过各种反应类型(包括环化,CH活化,双官能化,硝化,与手性配体的重排和不对称合成。硝酸铜的进一步改性,例如固体负载的硝酸铜或硝酸铜络合物,扩展了其在有机合成中的应用。本综述着重介绍了硝酸铜在有机合成中的最新进展及其机理。
更新日期:2018-03-14
中文翻译:

硝酸铜:有机合成的特权试剂
铜已被认为是替代有机合成中贵金属的理想候选者,特别是对于大规模实践而言。最近几十年见证了铜催化的和铜介导的有机反应的复兴和改善。硝酸铜是一种常见的无机铜盐,由于其商业上的可获得性,稳定性,廉价和对环境有益的性质,已被证明是有机合成中普遍存在的反应物。硝酸铜可用作硝化试剂,氧化剂,催化剂或促进剂,以及路易斯酸。值得注意的是,通过各种反应类型(包括环化,CH活化,双官能化,硝化,与手性配体的重排和不对称合成。硝酸铜的进一步改性,例如固体负载的硝酸铜或硝酸铜络合物,扩展了其在有机合成中的应用。本综述着重介绍了硝酸铜在有机合成中的最新进展及其机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号