当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Two Discrete RuCp* (Cp*=Pentamethylcyclopentadienyl) Binding Modes of N‐Confused Porphyrins: Peripheral π Complex and Sitting Atop Ruthenocenophane Complex by Skeletal Transformation
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-04-16 , DOI: 10.1002/chem.201801237 Takaaki Yamamoto 1 , Koki Mitsuno 1 , Shigeki Mori 2 , Shuhei Itoyama 3 , Yoshihito Shiota 3 , Kazunari Yoshizawa 3 , Masatoshi Ishida 1 , Hiroyuki Furuta 1
Chemistry - A European Journal ( IF 4.3 ) Pub Date : 2018-04-16 , DOI: 10.1002/chem.201801237 Takaaki Yamamoto 1 , Koki Mitsuno 1 , Shigeki Mori 2 , Shuhei Itoyama 3 , Yoshihito Shiota 3 , Kazunari Yoshizawa 3 , Masatoshi Ishida 1 , Hiroyuki Furuta 1
Affiliation
|
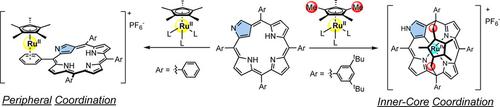
Complexation of a RuCp* cation with N‐confused tetraarylporphyrins (NCPs) forms directly bound ruthenium(II) pentamethylcyclopentadienyl (Cp*) π‐complex on a specific meso‐aryl group (e.g., phenyl) neighboring peripheral imino nitrogen of NCPs in high yields. In contrast, in the case of NCPs bearing bulky meso‐substituents (e.g., 3,5‐di‐tert‐butylphenyl), new ruthenocenophane‐like complex embedded on an N‐confused calix[4]phyrin was formed through multiple C−H bond activation of methyl groups of Cp* ligand. The mechanistic insight into the formation of the ruthenocenophane was derived from DFT calculations.
中文翻译:

N混淆的卟啉的两种离散RuCp *(Cp * =五甲基环戊二烯基)结合模式:外围π络合物和通过骨架转化位于钌烯十一烷复合物上
RuCp *阳离子与N-混淆的四芳基卟啉(NCPs)的络合可在NCPs的周边亚氨基氮的特定内消旋芳基(例如苯基)上与直接键合的钌(II)五甲基环戊二烯基(Cp *)π-络合物形成高产量。相比之下,在带有大体积内消旋取代基(例如3,5-二叔丁基苯基)的NCP的情况下,嵌入N混淆的杯[4]卟啉中的新的钌钌甲壳烷样复合物是通过多个CH形成的Cp *配体的甲基的键活化。从DFT计算中得出了钌钌甲壳烷形成机理的见解。
更新日期:2018-04-16
中文翻译:

N混淆的卟啉的两种离散RuCp *(Cp * =五甲基环戊二烯基)结合模式:外围π络合物和通过骨架转化位于钌烯十一烷复合物上
RuCp *阳离子与N-混淆的四芳基卟啉(NCPs)的络合可在NCPs的周边亚氨基氮的特定内消旋芳基(例如苯基)上与直接键合的钌(II)五甲基环戊二烯基(Cp *)π-络合物形成高产量。相比之下,在带有大体积内消旋取代基(例如3,5-二叔丁基苯基)的NCP的情况下,嵌入N混淆的杯[4]卟啉中的新的钌钌甲壳烷样复合物是通过多个CH形成的Cp *配体的甲基的键活化。从DFT计算中得出了钌钌甲壳烷形成机理的见解。


























 京公网安备 11010802027423号
京公网安备 11010802027423号