当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Formic Acid or Formate Derivatives as the In Situ Hydrogen Source in Au‐Catalyzed Reduction of para‐Chloronitrobenzene
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-03-13 , DOI: 10.1002/slct.201702863 Zhun Hu 1 , Shunquan Tan 1 , Rongli Mi 1 , Xiang Li 1 , Jing Bai 1 , Xiaoyan Guo 1 , Guoyang Hu 1 , Peng Hang 1 , Juan Li 1 , Dan Li 1 , Yang Yang 2 , Xianghui Yan 3
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-03-13 , DOI: 10.1002/slct.201702863 Zhun Hu 1 , Shunquan Tan 1 , Rongli Mi 1 , Xiang Li 1 , Jing Bai 1 , Xiaoyan Guo 1 , Guoyang Hu 1 , Peng Hang 1 , Juan Li 1 , Dan Li 1 , Yang Yang 2 , Xianghui Yan 3
Affiliation
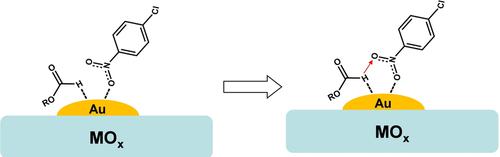
|
Formic acid and formate derivatives as hydrogen source on the hydrogenation of para‐chloronitrobenzene (p‐CNB) over supported gold catalysts were investigated. The hydrogenation activity in various formate derivatives followed the order: HCOOH < HCOONa < HCOOK < HCOONH4, which was in accordance with the decrement of electronegativity of cation species in formate derivatives. Moreover, in the case of HCOONH4 as hydrogen source, the p‐CNB conversion could enhance ten‐fold as the hydrogen source of H2 at 60 oC. This promotion effect was also found in other supported Au catalysts. These results provide a general alternative hydrogen source to replace conventional H2 as reducing agent for fine chemical processes.
中文翻译:

甲酸或甲酸酯衍生物作为原位氢源在Au催化还原对氯硝基苯中的作用
研究了在负载型金催化剂上对氯硝基苯(p - CNB)加氢时甲酸和甲酸衍生物作为氢源。各种甲酸盐衍生物中的氢化活性遵循以下顺序:HCOOH <HCOONa <HCOOK <HCOONH 4,这与甲酸盐衍生物中阳离子物种的电负性的降低有关。此外,在以HCOONH 4为氢源的情况下,在60 o C下,p- CNB转化率可以作为H 2的氢源提高十倍。在其他负载型Au催化剂中也发现了这种促进作用。这些结果提供了替代常规H 2的通用替代氢源。 作为精细化工过程的还原剂。
更新日期:2018-03-13
中文翻译:

甲酸或甲酸酯衍生物作为原位氢源在Au催化还原对氯硝基苯中的作用
研究了在负载型金催化剂上对氯硝基苯(p - CNB)加氢时甲酸和甲酸衍生物作为氢源。各种甲酸盐衍生物中的氢化活性遵循以下顺序:HCOOH <HCOONa <HCOOK <HCOONH 4,这与甲酸盐衍生物中阳离子物种的电负性的降低有关。此外,在以HCOONH 4为氢源的情况下,在60 o C下,p- CNB转化率可以作为H 2的氢源提高十倍。在其他负载型Au催化剂中也发现了这种促进作用。这些结果提供了替代常规H 2的通用替代氢源。 作为精细化工过程的还原剂。


























 京公网安备 11010802027423号
京公网安备 11010802027423号