当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Targeting Ligandable Pockets on Plant Homeodomain (PHD) Zinc Finger Domains by a Fragment-Based Approach.
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-20 , DOI: 10.1021/acschembio.7b01093 Anastasia Amato 1 , Xavier Lucas 1 , Alessio Bortoluzzi 1 , David Wright 1 , Alessio Ciulli 1
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-20 , DOI: 10.1021/acschembio.7b01093 Anastasia Amato 1 , Xavier Lucas 1 , Alessio Bortoluzzi 1 , David Wright 1 , Alessio Ciulli 1
Affiliation
|
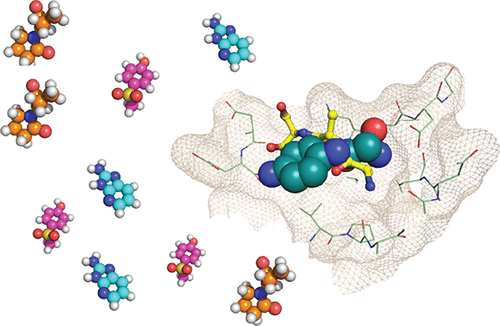
Plant homeodomain (PHD) zinc fingers are histone reader domains that are often associated with human diseases. Despite this, they constitute a poorly targeted class of readers, suggesting low ligandability. Here, we describe a successful fragment-based campaign targeting PHD fingers from the proteins BAZ2A and BAZ2B as model systems. We validated a pool of in silico fragments both biophysically and structurally and solved the first crystal structures of PHD zinc fingers in complex with fragments bound to an anchoring pocket at the histone binding site. The best-validated hits were found to displace a histone H3 tail peptide in competition assays. This work identifies new chemical scaffolds that provide suitable starting points for future ligand optimization using structure-guided approaches. The demonstrated ligandability of the PHD reader domains could pave the way for the development of chemical probes to drug this family of epigenetic readers.
中文翻译:

通过基于片段的方法靶向植物同源域 (PHD) 锌指域上的可配体口袋。
植物同源域 (PHD) 锌指是通常与人类疾病相关的组蛋白阅读器域。尽管如此,它们构成了一个目标性较差的读者类别,表明配体能力低。在这里,我们描述了一个成功的基于片段的活动,该活动针对来自蛋白质 BAZ2A 和 BAZ2B 的 PHD 手指作为模型系统。我们在生物物理和结构上验证了一组计算机碎片,并解决了 PHD 锌指的第一个晶体结构,其中碎片与组蛋白结合位点的锚定袋结合。发现经过最佳验证的命中在竞争测定中取代了组蛋白 H3 尾肽。这项工作确定了新的化学支架,为使用结构引导方法的未来配体优化提供了合适的起点。
更新日期:2018-03-12
中文翻译:

通过基于片段的方法靶向植物同源域 (PHD) 锌指域上的可配体口袋。
植物同源域 (PHD) 锌指是通常与人类疾病相关的组蛋白阅读器域。尽管如此,它们构成了一个目标性较差的读者类别,表明配体能力低。在这里,我们描述了一个成功的基于片段的活动,该活动针对来自蛋白质 BAZ2A 和 BAZ2B 的 PHD 手指作为模型系统。我们在生物物理和结构上验证了一组计算机碎片,并解决了 PHD 锌指的第一个晶体结构,其中碎片与组蛋白结合位点的锚定袋结合。发现经过最佳验证的命中在竞争测定中取代了组蛋白 H3 尾肽。这项工作确定了新的化学支架,为使用结构引导方法的未来配体优化提供了合适的起点。


























 京公网安备 11010802027423号
京公网安备 11010802027423号