当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Tandem Synthesis of 3,4‐Disubstituted Pyrroles from Aldehydes, 1,3‐Diketones and TosMIC Under Metal‐Free Conditions
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201800110 Kesari Lakshmi Manasa 1, 2 , Kasinathuni Naga Visweswara Sastry 1, 2 , Yellaiah Tangella 2 , Bathini Nagendra Babu 1, 2
ChemistrySelect ( IF 2.1 ) Pub Date : 2018-03-12 , DOI: 10.1002/slct.201800110 Kesari Lakshmi Manasa 1, 2 , Kasinathuni Naga Visweswara Sastry 1, 2 , Yellaiah Tangella 2 , Bathini Nagendra Babu 1, 2
Affiliation
|
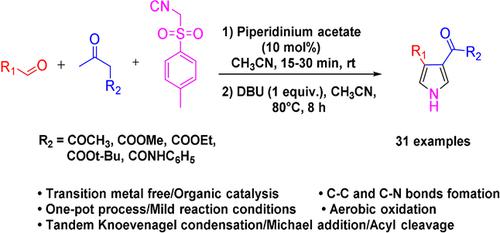
The method described herein is an efficient and simple process to obtain 3,4‐disubstituted pyrroles. This protocol utilizes the three component reaction of aldehydes, 1,3‐dicarbonyls and TosMIC in one pot under metal free conditions with moderate to excellent yields. The mechanism of the reaction involves a Knoevenagel condensation between an aldehyde and dicarbonyl substrate to give unsaturated alkene intermediate which further undergoes Michael addition with TosMIC to provide desired 3,4‐disubstituted pyrroles. The scope of the substrate was then explored to understand the diversity of functional group tolerance. A series of 31 examples were generated using the present protocol.
中文翻译:

在无金属条件下由醛,1,3-二酮和TosMIC串联合成3,4-二取代的吡咯
本文所述方法是获得3,4-二取代吡咯的一种有效且简单的方法。该方案在无金属的条件下,在一个锅中利用醛,1,3-二羰基化合物和TosMIC的三组分反应,具有中等至极好的收率。反应机理涉及醛和二羰基底物之间的Knoevenagel缩合反应,生成不饱和烯烃中间体,然后进一步与TosMIC进行迈克尔加成反应,得到所需的3,4-二取代吡咯。然后探索底物的范围以了解官能团耐受性的多样性。使用本协议生成了一系列31个示例。
更新日期:2018-03-12
中文翻译:

在无金属条件下由醛,1,3-二酮和TosMIC串联合成3,4-二取代的吡咯
本文所述方法是获得3,4-二取代吡咯的一种有效且简单的方法。该方案在无金属的条件下,在一个锅中利用醛,1,3-二羰基化合物和TosMIC的三组分反应,具有中等至极好的收率。反应机理涉及醛和二羰基底物之间的Knoevenagel缩合反应,生成不饱和烯烃中间体,然后进一步与TosMIC进行迈克尔加成反应,得到所需的3,4-二取代吡咯。然后探索底物的范围以了解官能团耐受性的多样性。使用本协议生成了一系列31个示例。



























 京公网安备 11010802027423号
京公网安备 11010802027423号