当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Peptide Retention in Hydrophilic Strong Anion Exchange Chromatography Is Driven by Charged and Aromatic Residues.
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-03-21 , DOI: 10.1021/acs.analchem.7b05157 Sven H Giese 1 , Yasushi Ishihama 2 , Juri Rappsilber 1, 2, 3
Analytical Chemistry ( IF 7.4 ) Pub Date : 2018-03-21 , DOI: 10.1021/acs.analchem.7b05157 Sven H Giese 1 , Yasushi Ishihama 2 , Juri Rappsilber 1, 2, 3
Affiliation
|
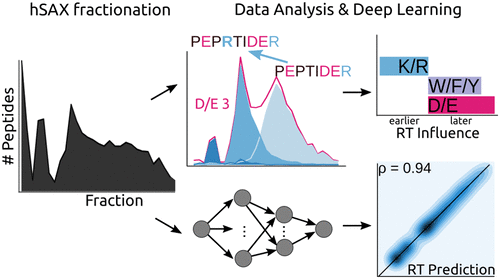
Hydrophilic strong anion exchange chromatography (hSAX) is becoming a popular method for the prefractionation of proteomic samples. However, the use and further development of this approach is affected by the limited understanding of its retention mechanism and the absence of elution time prediction. Using a set of 59 297 confidentially identified peptides, we performed an explorative analysis and built a predictive deep learning model. As expected, charged residues are the major contributors to the retention time through electrostatic interactions. Aspartic acid and glutamic acid have a strong retaining effect and lysine and arginine have a strong repulsion effect. In addition, we also find the involvement of aromatic amino acids. This suggests a substantial contribution of cation-π interactions to the retention mechanism. The deep learning approach was validated using 5-fold cross-validation (CV) yielding a mean prediction accuracy of 70% during CV and 68% on a hold-out validation set. The results of this study emphasize that not only electrostatic interactions but rather diverse types of interactions must be integrated to build a reliable hSAX retention time predictor.
中文翻译:

亲水性强阴离子交换色谱中的肽保留由带电和芳香族残留物驱动。
亲水强阴离子交换色谱 (hSAX) 正成为蛋白质组样品预分馏的流行方法。然而,这种方法的使用和进一步发展受到对其保留机制的有限理解和缺乏洗脱时间预测的影响。使用一组 59 297 个机密识别的肽,我们进行了探索性分析并构建了预测性深度学习模型。正如预期的那样,带电残留物是通过静电相互作用导致保留时间的主要因素。天冬氨酸和谷氨酸有很强的保留作用,赖氨酸和精氨酸有很强的排斥作用。此外,我们还发现了芳香族氨基酸的参与。这表明阳离子-π 相互作用对保留机制的重要贡献。深度学习方法使用 5 折交叉验证 (CV) 进行验证,在 CV 期间产生 70% 的平均预测准确度,在保留验证集上产生 68%。这项研究的结果强调,不仅必须整合静电相互作用,而且还必须整合多种类型的相互作用,以构建可靠的 hSAX 保留时间预测器。
更新日期:2018-03-12
中文翻译:

亲水性强阴离子交换色谱中的肽保留由带电和芳香族残留物驱动。
亲水强阴离子交换色谱 (hSAX) 正成为蛋白质组样品预分馏的流行方法。然而,这种方法的使用和进一步发展受到对其保留机制的有限理解和缺乏洗脱时间预测的影响。使用一组 59 297 个机密识别的肽,我们进行了探索性分析并构建了预测性深度学习模型。正如预期的那样,带电残留物是通过静电相互作用导致保留时间的主要因素。天冬氨酸和谷氨酸有很强的保留作用,赖氨酸和精氨酸有很强的排斥作用。此外,我们还发现了芳香族氨基酸的参与。这表明阳离子-π 相互作用对保留机制的重要贡献。深度学习方法使用 5 折交叉验证 (CV) 进行验证,在 CV 期间产生 70% 的平均预测准确度,在保留验证集上产生 68%。这项研究的结果强调,不仅必须整合静电相互作用,而且还必须整合多种类型的相互作用,以构建可靠的 hSAX 保留时间预测器。



























 京公网安备 11010802027423号
京公网安备 11010802027423号