Applied Catalysis B: Environment and Energy ( IF 22.1 ) Pub Date : 2018-03-11 , DOI: 10.1016/j.apcatb.2018.03.028 Ana B. Calcerrada , Ana R. de la Osa , Javier Llanos , Fernando Dorado , Antonio de Lucas-Consuegra
|
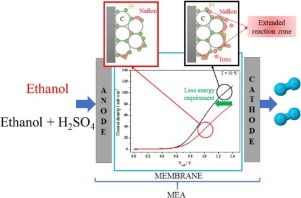
The addition of H2SO4 to the anolyte feeding solution for the electrochemical reforming of ethanol has been studied in terms of activity and stability for hydrogen production in a PEM electrolysis cell. Five equivalent MEAs based on commercial Pt-Sn/C and Pt/C as anode and cathode materials, respectively, and a Nafion membrane, have been prepared and tested to optimize the addition of the H2SO4. Results demonstrated that the addition of an optimal H2SO4 concentration (ethanol 4 mol L−1 and 0.01 mol L−1 H2SO4) enhances the electrocatalytic activity and stability of the MEA by decreasing the energy requirements for hydrogen production. According to impedance spectroscopy experiments, this improvement is caused by a decrease in the anodic charge transfer resistance, probably due to an increase in the number of contact triple points (electrolyte/catalyst particles/electronic support). However, the addition of a higher H2SO4 concentration (0.05 mol L−1) induced the dissolution of the Pt-Sn/C anodic catalyst causing a severe degradation of the MEA. Therefore, the optimal addition of H2SO4, which is not consumed during the electrochemical reforming experiments, may be of great practical importance for using this technology for the renewable hydrogen production from real bioethanol streams.
中文翻译:

加硫酸辅助乙醇电化学重整制氢
就在PEM电解池中制氢的活性和稳定性方面,已经研究了将H 2 SO 4添加到阳极液进料溶液中以进行乙醇的电化学重整。已经制备并测试了五个分别基于市售Pt-Sn / C和Pt / C作为阳极和阴极材料以及Nafion膜的等效MEA,以优化H 2 SO 4的添加。结果表明,添加了最佳的H 2 SO 4浓度(乙醇4 mol L -1和0.01 mol L -1 H 2 SO 4)通过降低制氢所需的能量来增强MEA的电催化活性和稳定性。根据阻抗谱实验,这种改善是由于阳极电荷转移电阻的降低而引起的,这可能是由于接触三点(电解质/催化剂颗粒/电子载体)数量增加所致。但是,添加更高的H 2 SO 4浓度(0.05mol L -1)引起Pt-Sn / C阳极催化剂的溶解,导致MEA的严重降解。因此,H 2 SO 4的最佳添加在电化学重整实验中不消耗的碳,对于将这种技术用于从实际的生物乙醇流中生产可再生氢具有非常重要的现实意义。


























 京公网安备 11010802027423号
京公网安备 11010802027423号