当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
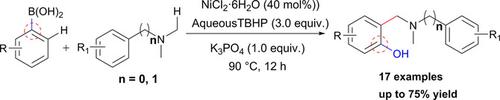
Nickel Catalyzed Ipso‐hydroxylation and Subsequent Cross Dehydrogenative Coupling of Arylboronic Acids with Tertiary Amines: A Facile Access to α‐phenolated Tertiary Amines
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-03-25 , DOI: 10.1002/adsc.201701625 Promod Kumar 1 , Anup Kumar Sharma 1 , Rahul Singh 1 , Tirumaleswararao Guntreddi 1 , Krishna Nand Singh 1
Advanced Synthesis & Catalysis ( IF 5.4 ) Pub Date : 2018-03-25 , DOI: 10.1002/adsc.201701625 Promod Kumar 1 , Anup Kumar Sharma 1 , Rahul Singh 1 , Tirumaleswararao Guntreddi 1 , Krishna Nand Singh 1
Affiliation
|
A straightforward and new approach has been developed for the α‐functionalization of tertiary amines via sequential oxidative hydoxylation and cross‐dehydrogenative coupling (CDC) of arylboronic acids with tertiary amines to afford α‐phenolated tertiary amines. The results demonstrate an easy arylation of C(sp3)−H bond in the presence of an inexpensive and readily available nickel metal salt.
中文翻译:

镍催化的异丙基羟基化和随后的芳硼酸与叔胺的交叉脱氢偶联:轻松获得α-酚化叔胺
通过芳基硼酸与叔胺的顺序氧化羟基化和交叉脱氢偶联(CDC),开发了一种简单,新颖的方法,用于叔胺的α-官能化,从而制得α-酚化叔胺。结果表明,在存在廉价且易于获得的镍金属盐的情况下,C(sp 3)-H键易于芳基化。
更新日期:2018-03-25
中文翻译:

镍催化的异丙基羟基化和随后的芳硼酸与叔胺的交叉脱氢偶联:轻松获得α-酚化叔胺
通过芳基硼酸与叔胺的顺序氧化羟基化和交叉脱氢偶联(CDC),开发了一种简单,新颖的方法,用于叔胺的α-官能化,从而制得α-酚化叔胺。结果表明,在存在廉价且易于获得的镍金属盐的情况下,C(sp 3)-H键易于芳基化。


























 京公网安备 11010802027423号
京公网安备 11010802027423号