Journal of Solid State Chemistry ( IF 3.3 ) Pub Date : 2018-03-07 , DOI: 10.1016/j.jssc.2018.03.002 Florian Ledderboge , Jan Nowak , Hans-Joachim Massonne , Katharina Förg , Henning A. Höppe , Thomas Schleid
|
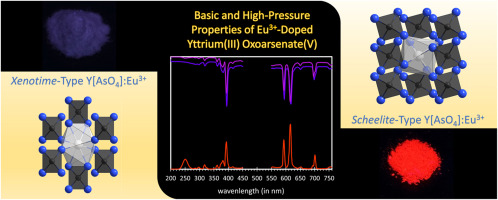
Colourless, water- and air-stable single crystals of yttrium(III) oxoarsenate(V) Y[AsO4] in the xenotime-type crystal structure were prepared by the reaction of yttrium sesquioxide (Y2O3) dissolved in aqueous nitric acid (13%) with a solution of arsenic(V) oxide hydrate (As2O5 ∙ 3 H2O) and subsequent neutralization with 1 M caustic soda. Y[AsO4] crystallizes tetragonally in the space group I41/amd with the lattice parameters a = 704.63(6) and c = 628.94(5) pm for Z = 4 and is isotypic to the minerals xenotime RE[PO4] (RE: mainly Y and Yb) and chernovite RE[AsO4] (RE: mainly Y and Ce). This xenotime-type yttrium compound was used as precursor in a high-pressure experiment (20 kbar) at 700 °C to create a new tetragonal modification of Y[AsO4]. It shows the scheelite-type structure (space group: I41/a) with the lattice parameters a = 498.23(4) and c = 1120.71(9) pm for Z = 4, named after the mineral scheelite (Ca[WO4]). Both tetragonal structures are characterized by only one crystallographically unique position for each of the Y3+, As5+ and O2– ions with distances of d(Y–O) = 232 and 241 pm (C.N. = 8) as well as d(As–O) = 169 pm (C.N. = 4) in the case of the scheelite-type structure. The xenotime-type compound shows an unexpected slight decrease in average bond lengths for the yttrium to oxygen (d(Y–O) = 230 and 241 pm, C.N. = 8) as well as for the arsenic to oxygen distances (d(As–O) = 168 pm, C.N. = 4), accompanied by a drastic density increase from Dx = 4.85 (xenotime type) to Dx = 5.44 g ∙ cm–3 (scheelite type). Luminescence spectroscopic measurements of the Eu3+-doped Y[AsO4] samples, obtained in experiments at similar conditions as for the pure compounds, show a bright, reddish lighting for the scheelite type, which does not occur for the xenotime type of yttrium(III) oxoarsenate(V).
中文翻译:

含氧砷酸钇(III)的高压研究:Xenotime型前驱体掺杂的Eu 3+掺杂的白钨矿型Y [AsO 4 ]的晶体结构和发光性能
无色,拒水和钇的空气稳定的单晶(III)oxoarsenate(V)Y [ASO 4 ]在磷钇矿-类型晶体结构通过的三氧化二钇(Y反应制得2 ö 3溶解于硝酸水溶液) (13%)用三水合氧化砷(As 2 O 5 ∙3 H 2 O)溶液处理,然后用1 M苛性钠中和。Y [AsO 4 ]在空间组I 4 1 / amd中四方结晶,Z的晶格参数a = 704.63(6)和c = 628.94(5)pm= 4,与矿物质xenotime RE [PO 4 ](RE:主要是Y和Yb)和钙长石RE [AsO 4 ](RE:主要是Y和Ce)同型。在700°C的高压实验(20 kbar)中,将这种Xenotime型钇化合物用作前体,以产生Y [AsO 4 ]的新的四边形修饰。它显示了白锌矿型结构(空间群:I 4 1 / a),Z = 4时具有晶格参数a = 498.23(4)和c = 1120.71(9)pm,以矿物白钨矿(Ca [WO 4])。这两个四方结构的特征是每个Y 3+,As 5+和O 2–离子只有一个晶体学上唯一的位置,距离d(Y–O)= 232和241 pm(CN = 8)以及d白钨矿型结构时(As–O)= 169 pm(CN = 4)。异种时间型化合物对钇与氧的平均键长(d(Y–O)= 230和241 pm,CN = 8)以及砷与氧的距离(d(As– O)= 168 pm,CN = 4),伴随着密度急剧增加,从D x = 4.85(xenotime类型)增加到D x= 5.44 g∙cm –3(白钨矿型)。在与纯化合物相似的条件下进行的实验中获得的Eu 3+掺杂Y [AsO 4 ]样品的发光光谱测量结果显示,白钨矿类型的辉光发红光,钇的钇矿类型却不发生这种光。 (III)含氧砷酸盐(V)。



























 京公网安备 11010802027423号
京公网安备 11010802027423号