当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
In Vitro, in Silico, and in Vivo Assessments of Intestinal Precipitation and Its Impact on Bioavailability of a BCS Class 2 Basic Compound
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01143 Dawen Kou , Chen Zhang 1 , Hiuwing Yiu , Tania Ng , Joseph W. Lubach , Matthew Janson , Chen Mao , Matthew Durk , Leslie Chinn , Helen Winter , Larry Wigman , Peter Yehl
Molecular Pharmaceutics ( IF 4.9 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acs.molpharmaceut.7b01143 Dawen Kou , Chen Zhang 1 , Hiuwing Yiu , Tania Ng , Joseph W. Lubach , Matthew Janson , Chen Mao , Matthew Durk , Leslie Chinn , Helen Winter , Larry Wigman , Peter Yehl
Affiliation
|
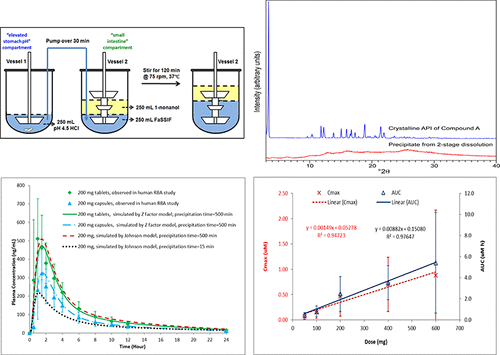
In this study, a multipronged approach of in vitro experiments, in silico simulations, and in vivo studies was developed to evaluate the dissolution, supersaturation, precipitation, and absorption of three formulations of Compound-A, a BCS class 2 weak base with pH-dependent solubility. In in vitro 2-stage dissolution experiments, the solutions were highly supersaturated with no precipitation at the low dose but increasing precipitation at higher doses. No difference in precipitation was observed between the capsules and tablets. The in vitro precipitate was found to be noncrystalline with higher solubility than the crystalline API, and was readily soluble when the drug concentration was lowered by dilution. A gastric transit and biphasic dissolution (GTBD) model was developed to better mimic gastric transfer and intestinal absorption. Precipitation was also observed in GTBD, but the precipitate redissolved and partitioned into the organic phase. In vivo data from the phase 1 clinical trial showed linear and dose proportional PK for the formulations with no evidence of in vivo precipitation. While the in vitro precipitation observed in the 2-stage dissolution appeared to overestimate in vivo precipitation, the GTBD model provided absorption profiles consistent with in vivo data. In silico simulation of plasma concentrations by GastroPlus using biorelevant in vitro dissolution data from the tablets and capsules and assuming negligible precipitation was in line with the observed in vivo profiles of the two formulations. The totality of data generated with Compound-A indicated that the bioavailability differences among the three formulations were better explained by the differences in gastric dissolution than intestinal precipitation. The lack of intestinal precipitation was consistent with several other BCS class 2 basic compounds in the literature for which highly supersaturated concentrations and rapid absorption were also observed.
中文翻译:

在体外,硅铝,并在体内肠道降水的评估和BCS 2类碱性化合物的生物利用度及其影响
在这项研究中,开发了一种多方面的体外实验,计算机模拟和体内研究方法来评估化合物A的三种制剂的溶解,过饱和,沉淀和吸收,这是一种BCS 2类弱碱,pH-依赖的溶解度。在体外两阶段溶出度实验中,溶液高度过饱和,低剂量时无沉淀,高剂量时沉淀增加。在胶囊和片剂之间未观察到沉淀的差异。在体外发现该沉淀是非结晶的,具有比结晶API更高的溶解度,并且当通过稀释降低药物浓度时很容易溶解。胃转运和双相溶出(GTBD)模型被开发来更好地模拟胃转移和肠道吸收。在GTBD中也观察到沉淀,但沉淀重新溶解并分配到有机相中。来自1期临床试验的体内数据显示,该制剂的线性和剂量比例PK没有体内沉淀的迹象。虽然在两阶段溶出中观察到的体外沉淀似乎高估了体内沉淀,但GTBD模型提供的吸收曲线与体内数据。使用来自片剂和胶囊的生物相关的体外溶出数据,通过GastroPlus对血浆浓度进行计算机模拟,并假设沉淀量可忽略不计,这与两种制剂的体内观察结果一致。用化合物-A生成的数据总数表明,三种制剂之间的生物利用度差异可以通过胃溶解度的差异而不是肠道沉淀来更好地解释。肠道沉淀的缺乏与文献中其他几种BCS 2类碱性化合物一致,在这些化合物中也观察到了高度过饱和的浓度和快速吸收。
更新日期:2018-03-09
中文翻译:

在体外,硅铝,并在体内肠道降水的评估和BCS 2类碱性化合物的生物利用度及其影响
在这项研究中,开发了一种多方面的体外实验,计算机模拟和体内研究方法来评估化合物A的三种制剂的溶解,过饱和,沉淀和吸收,这是一种BCS 2类弱碱,pH-依赖的溶解度。在体外两阶段溶出度实验中,溶液高度过饱和,低剂量时无沉淀,高剂量时沉淀增加。在胶囊和片剂之间未观察到沉淀的差异。在体外发现该沉淀是非结晶的,具有比结晶API更高的溶解度,并且当通过稀释降低药物浓度时很容易溶解。胃转运和双相溶出(GTBD)模型被开发来更好地模拟胃转移和肠道吸收。在GTBD中也观察到沉淀,但沉淀重新溶解并分配到有机相中。来自1期临床试验的体内数据显示,该制剂的线性和剂量比例PK没有体内沉淀的迹象。虽然在两阶段溶出中观察到的体外沉淀似乎高估了体内沉淀,但GTBD模型提供的吸收曲线与体内数据。使用来自片剂和胶囊的生物相关的体外溶出数据,通过GastroPlus对血浆浓度进行计算机模拟,并假设沉淀量可忽略不计,这与两种制剂的体内观察结果一致。用化合物-A生成的数据总数表明,三种制剂之间的生物利用度差异可以通过胃溶解度的差异而不是肠道沉淀来更好地解释。肠道沉淀的缺乏与文献中其他几种BCS 2类碱性化合物一致,在这些化合物中也观察到了高度过饱和的浓度和快速吸收。



























 京公网安备 11010802027423号
京公网安备 11010802027423号