当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Exploring the Binding Mechanism of Metabotropic Glutamate Receptor 5 Negative Allosteric Modulators in Clinical Trials by Molecular Dynamics Simulations
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acschemneuro.8b00059 Tingting Fu 1, 2 , Guoxun Zheng 1, 2 , Gao Tu 1, 2 , Fengyuan Yang 1, 2 , Yuzong Chen 3 , Xiaojun Yao 4 , Xiaofeng Li 1, 2 , Weiwei Xue 1 , Feng Zhu 1, 2
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2018-03-09 00:00:00 , DOI: 10.1021/acschemneuro.8b00059 Tingting Fu 1, 2 , Guoxun Zheng 1, 2 , Gao Tu 1, 2 , Fengyuan Yang 1, 2 , Yuzong Chen 3 , Xiaojun Yao 4 , Xiaofeng Li 1, 2 , Weiwei Xue 1 , Feng Zhu 1, 2
Affiliation
|
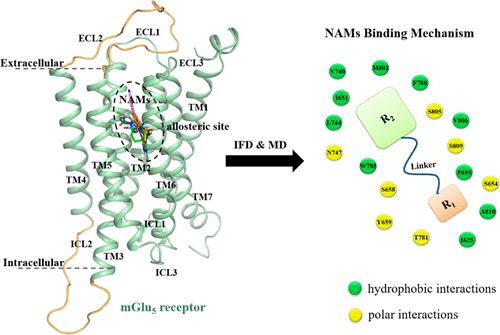
Metabotropic glutamate receptor 5 (mGlu5) plays a key role in synaptic information storage and memory, which is a well-known target for a variety of psychiatric and neurodegenerative disorders. In recent years, the increasing efforts have been focused on the design of allosteric modulators, and the negative allosteric modulators (NAMs) are the front-runners. Recently, the architecture of the transmembrane (TM) domain of mGlu5 receptor has been determined by crystallographic experiment. However, it has been not well understood how the pharmacophores of NAMs accommodated into the allosteric binding site. In this study, molecular dynamics (MD) simulations were performed on mGlu5 receptor bound with NAMs in preclinical or clinical development to shed light on this issue. In order to identify the key residues, the binding free energies as well as per-residue contributions for NAMs binding to mGlu5 receptor were calculated. Subsequently, the in silico site-directed mutagenesis of the key residues was performed to verify the accuracy of simulation models. As a result, the shared common features of the studied 5 clinically important NAMs (mavoglurant, dipraglurant, basimglurant, STX107, and fenobam) interacting with 11 residues in allosteric site were obtained. This comprehensive study presented a better understanding of mGlu5 receptor NAMs binding mechanism, which would be further used as a useful framework to assess and discover novel lead scaffolds for NAMs.
中文翻译:

通过分子动力学模拟探索代谢型谷氨酸受体5负变构调节剂的结合机理。
代谢型谷氨酸受体5(mGlu 5)在突触信息的存储和记忆中起关键作用,突触信息是各种精神病和神经退行性疾病的众所周知的靶标。近年来,越来越多的努力集中在变构调节剂的设计上,而负变构调节剂(NAM)处于领先地位。最近,已经通过晶体学实验确定了mGlu 5受体的跨膜(TM)结构域的体系结构。但是,尚未完全了解NAM的药效基团如何适应变构结合位点。在这项研究中,对mGlu 5进行了分子动力学(MD)模拟在临床前或临床开发中将受体与NAM结合以阐明这一问题。为了鉴定关键残基,计算了与mGlu 5受体结合的NAM的结合自由能以及每个残基的贡献。随后,对关键残基进行了计算机定点诱变,以验证模拟模型的准确性。结果,获得了与5个变构位点中的11个残基相互作用的5个临床上重要的NAM(强效剂,双精胺剂,basimglurant,STX107和fenobam)的共有共同特征。这项全面的研究提出了对mGlu 5的更好理解 受体NAM结合机制,将其进一步用作评估和发现NAM的新型铅支架的有用框架。
更新日期:2018-03-09
中文翻译:

通过分子动力学模拟探索代谢型谷氨酸受体5负变构调节剂的结合机理。
代谢型谷氨酸受体5(mGlu 5)在突触信息的存储和记忆中起关键作用,突触信息是各种精神病和神经退行性疾病的众所周知的靶标。近年来,越来越多的努力集中在变构调节剂的设计上,而负变构调节剂(NAM)处于领先地位。最近,已经通过晶体学实验确定了mGlu 5受体的跨膜(TM)结构域的体系结构。但是,尚未完全了解NAM的药效基团如何适应变构结合位点。在这项研究中,对mGlu 5进行了分子动力学(MD)模拟在临床前或临床开发中将受体与NAM结合以阐明这一问题。为了鉴定关键残基,计算了与mGlu 5受体结合的NAM的结合自由能以及每个残基的贡献。随后,对关键残基进行了计算机定点诱变,以验证模拟模型的准确性。结果,获得了与5个变构位点中的11个残基相互作用的5个临床上重要的NAM(强效剂,双精胺剂,basimglurant,STX107和fenobam)的共有共同特征。这项全面的研究提出了对mGlu 5的更好理解 受体NAM结合机制,将其进一步用作评估和发现NAM的新型铅支架的有用框架。


























 京公网安备 11010802027423号
京公网安备 11010802027423号