Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-03-09 , DOI: 10.1016/j.bmc.2018.02.051 Stephane Jeanmart , Julien Gagnepain , Pulakesh Maity , Clemens Lamberth , Fredrik Cederbaum , Ramya Rajan , Olivier Jacob , Mathias Blum , Stephane Bieri
|
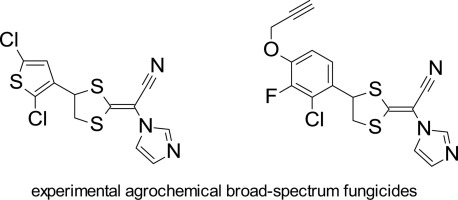
Novel imidazole-based ketene dithioacetals show impressive in planta activity against the economically important plant pathogens Alternaria solani, Botryotinia fuckeliana, Erysiphe necator and Zymoseptoria tritici. Especially derivatives of the topical antifungal lanoconazole, which bear an alkynyloxy or a heteroaryl group in the para-position of the phenyl ring, exhibit excellent control of the mentioned phytopathogens. These compounds inhibit 14α -demethylase in the sterol biosynthesis pathway of the fungi. Synthesis routes starting from either benzaldehydes or acetophenones as well as structure-activity relationships are discussed in detail.
中文翻译:

新型咪唑基乙烯酮二硫缩醛的合成及杀菌活性
新型咪唑类烯酮二硫表现出不俗的植物内对经济重要的植物病原体的活性链格孢菌,灰葡萄fuckeliana,葡萄白粉菌和Zymoseptoria小麦。特别是局部抗真菌拉诺康唑的衍生物,其在苯环的对位带有炔氧基或杂芳基,表现出对上述植物病原体的良好控制。这些化合物在真菌的固醇生物合成途径中抑制14α-脱甲基酶。详细讨论了从苯甲醛或苯乙酮开始的合成路线以及结构-活性关系。

























 京公网安备 11010802027423号
京公网安备 11010802027423号