当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Changing Face: A Key Residue for the Addition of Water by Sclareol Synthase
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acscatal.8b00121 Meirong Jia 1 , Terrence E. O’Brien 2 , Yue Zhang 2 , Justin B. Siegel 2, 3, 4 , Dean J. Tantillo 2 , Reuben J. Peters 1
ACS Catalysis ( IF 12.9 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acscatal.8b00121 Meirong Jia 1 , Terrence E. O’Brien 2 , Yue Zhang 2 , Justin B. Siegel 2, 3, 4 , Dean J. Tantillo 2 , Reuben J. Peters 1
Affiliation
|
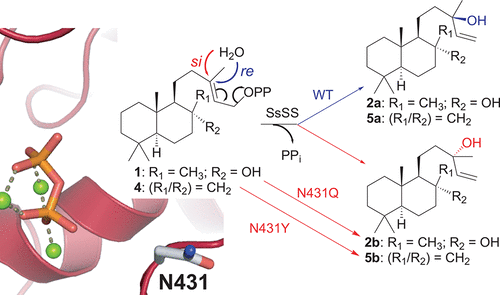
Sclareol synthase from Salvia sclarea (SsSS) naturally acts on 8α-hydroxy-copalyl diphosphate (1), stereoselectively adding water to produce (13R)-sclareol (2a), and similarly yields hydroxylated products with many-fold other such bicyclic diterpene precursors. Here, a key residue for this addition of water was identified. Strikingly, substitution with glutamine switches stereochemical outcome with 1, leading to the selective production of (13S)-sclareol (2b). Moreover, changes to the stereospecificity of water addition with the structurally closely related substrate copalyl diphosphate (4) could be accomplished with alternative substitutions. Thus, this approach is expected to provide biosynthetic access to both epimers of 13-hydroxylated derivatives of many-fold labdane-related diterpenes.
中文翻译:

换脸:香紫苏醇合酶添加水的主要残留物
丹参中的香紫苏醇合酶(SsSS)自然作用于8α-羟基-二甲基鲸蜡基二磷酸(1),立体选择性地加水生成(13 R)-香紫苏醇(2a),并类似地与其他双环二萜前体形成许多倍的羟基化产物。在此,确定了用于添加水的关键残留物。引人注目的是,用谷氨酰胺取代将立体化学结果切换为1,从而导致选择性生成(13 S)-香紫苏醇(2b)。此外,与结构密切相关的底物二磷酸钴基棕榈酸酯改变了加水的立体定向性(4)可以通过替代替换来实现。因此,预期该方法将提供生物合成途径,使之与许多与拉丹烷有关的二萜的13-羟基化衍生物的两个差向异构体发生生物合成。
更新日期:2018-03-08
中文翻译:

换脸:香紫苏醇合酶添加水的主要残留物
丹参中的香紫苏醇合酶(SsSS)自然作用于8α-羟基-二甲基鲸蜡基二磷酸(1),立体选择性地加水生成(13 R)-香紫苏醇(2a),并类似地与其他双环二萜前体形成许多倍的羟基化产物。在此,确定了用于添加水的关键残留物。引人注目的是,用谷氨酰胺取代将立体化学结果切换为1,从而导致选择性生成(13 S)-香紫苏醇(2b)。此外,与结构密切相关的底物二磷酸钴基棕榈酸酯改变了加水的立体定向性(4)可以通过替代替换来实现。因此,预期该方法将提供生物合成途径,使之与许多与拉丹烷有关的二萜的13-羟基化衍生物的两个差向异构体发生生物合成。



























 京公网安备 11010802027423号
京公网安备 11010802027423号