当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Photochemical Control of Protein Arginine Deiminase (PAD) Activity
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acschembio.8b00053 Santanu Mondal 1, 2 , Sangram S. Parelkar 1, 2 , Mitesh Nagar 1, 2 , Paul R. Thompson 1, 2
ACS Chemical Biology ( IF 4 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acschembio.8b00053 Santanu Mondal 1, 2 , Sangram S. Parelkar 1, 2 , Mitesh Nagar 1, 2 , Paul R. Thompson 1, 2
Affiliation
|
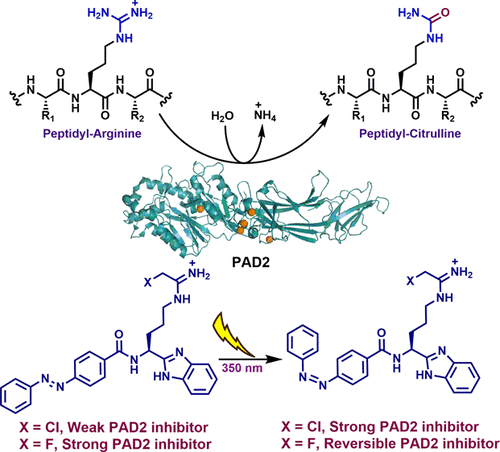
Protein arginine deiminases (PADs) play an important role in the pathogenesis of various diseases, including rheumatoid arthritis, multiple sclerosis, lupus, ulcerative colitis, and breast cancer. Therefore, the development of PAD inhibitors has drawn significant research interest in recent years. Herein, we describe the development of the first photoswitchable PAD inhibitors. These compounds possess an azobenzene photoswitch to optically control PAD activity. Screening of a series of inhibitors structurally similar to BB-Cl-amidine afforded compounds 1 and 2 as the most promising candidates for the light-controlled inhibition of PAD2; the cis isomer of 1 is 10-fold more potent than its trans isomer, whereas the trans isomer of 2 is 45-fold more potent than the corresponding cis isomer. The altered inhibitory potency upon photoisomerization has been confirmed in a competitive activity-based protein profiling (ABPP) assay. Further investigations indicate that the trans isomer of 2 is an irreversible inhibitor, whereas the cis isomer acts as a competitive inhibitor. In cells, the trans isomer of compound 1 is completely inactive, whereas the cis isomer inhibits histone H3-citrullination in a dose-dependent manner. Taken together, 1 serves as the foundation for developing photopharmaceuticals that can be activated at the desired tissue, using light, to treat diseases where PAD activity is dysregulated.
中文翻译:

蛋白质精氨酸脱亚氨酶(PAD)活性的光化学控制
精氨酸脱氨酶(PAD)在各种疾病的发病机理中起重要作用,包括类风湿性关节炎,多发性硬化症,狼疮,溃疡性结肠炎和乳腺癌。因此,近年来PAD抑制剂的开发引起了广泛的研究兴趣。在这里,我们描述了第一个光开关PAD抑制剂的发展。这些化合物具有偶氮苯光电开关,可以光学控制PAD活性。筛选一系列结构相似于BB-C1--的抑制剂,得到化合物1和2作为PAD2的光控抑制的最有希望的候选物。的顺式异构体1是10倍比其更有效反异构体,而2的反式异构体的效力是相应的顺式异构体的45倍。在基于竞争活性的蛋白质谱分析(ABPP)分析中已经证实了光异构化后抑制能力的改变。进一步的研究表明2的反式异构体是不可逆的抑制剂,而顺式异构体则是竞争性抑制剂。在细胞中,化合物1的反式异构体是完全无活性的,而顺式异构体则以剂量依赖的方式抑制组蛋白H3-瓜氨酸化。合计1 用作开发光药物的基础,该光药物可以使用光在所需的组织处激活,以治疗PAD活性异常的疾病。
更新日期:2018-03-08
中文翻译:

蛋白质精氨酸脱亚氨酶(PAD)活性的光化学控制
精氨酸脱氨酶(PAD)在各种疾病的发病机理中起重要作用,包括类风湿性关节炎,多发性硬化症,狼疮,溃疡性结肠炎和乳腺癌。因此,近年来PAD抑制剂的开发引起了广泛的研究兴趣。在这里,我们描述了第一个光开关PAD抑制剂的发展。这些化合物具有偶氮苯光电开关,可以光学控制PAD活性。筛选一系列结构相似于BB-C1--的抑制剂,得到化合物1和2作为PAD2的光控抑制的最有希望的候选物。的顺式异构体1是10倍比其更有效反异构体,而2的反式异构体的效力是相应的顺式异构体的45倍。在基于竞争活性的蛋白质谱分析(ABPP)分析中已经证实了光异构化后抑制能力的改变。进一步的研究表明2的反式异构体是不可逆的抑制剂,而顺式异构体则是竞争性抑制剂。在细胞中,化合物1的反式异构体是完全无活性的,而顺式异构体则以剂量依赖的方式抑制组蛋白H3-瓜氨酸化。合计1 用作开发光药物的基础,该光药物可以使用光在所需的组织处激活,以治疗PAD活性异常的疾病。



























 京公网安备 11010802027423号
京公网安备 11010802027423号