当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
How the Arrangement of Alkyl Substituents Affects the Stability of Delocalized Carbocations
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.joc.8b00415 Paul R. Rablen 1 , Nathalie A. Perry-Freer 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-08 00:00:00 , DOI: 10.1021/acs.joc.8b00415 Paul R. Rablen 1 , Nathalie A. Perry-Freer 1
Affiliation
|
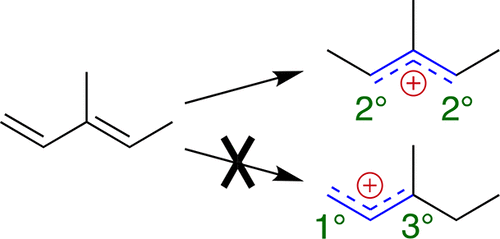
G-4 calculations are used to explore which carbon atoms of methylated butadienes, methylated cyclopentadienes, and methylated benzenes are most readily protonated to yield delocalized allyl and pentadienyl cations. While it is not surprising that alkylation of the positions bearing formal positive charge stabilizes these cations, several other effects are less obvious. First, alkylation of positions in the delocalized cation that do not bear formal charge is beneficial, to an extent about a quarter to a third as great as at charged positions. Second, alkylation of the position receiving the proton disfavors protonation. Finally, at least in the acyclic systems, the more symmetrical substitution pattern that is 2° at both ends is moderately preferred to the less symmetrical pattern that is 3° at one end and 1° at the other. Taking all three of these factors into account, as well as substitution at the formally charged centers, models the stability of all 94 delocalized cations quite well.
中文翻译:

烷基取代基的排列方式如何影响离域碳阳离子的稳定性
使用G-4计算来探索甲基化的丁二烯,甲基化的环戊二烯和甲基化的苯中哪个碳原子最容易质子化以生成离域的烯丙基和戊二烯基阳离子。虽然带有形式正电荷的位置的烷基化作用稳定了这些阳离子也就不足为奇了,但其他几种作用却不太明显。首先,不带正电荷的离域阳离子位置的烷基化是有益的,其程度约为带电荷位置的四分之一至三分之一。第二,接受质子的位置的烷基化不利于质子化。最后,至少在非循环系统中,两端对称为3°,另一端为1°的较不对称的模式适度偏向于两端为2°的较对称的取代模式。
更新日期:2018-03-08
中文翻译:

烷基取代基的排列方式如何影响离域碳阳离子的稳定性
使用G-4计算来探索甲基化的丁二烯,甲基化的环戊二烯和甲基化的苯中哪个碳原子最容易质子化以生成离域的烯丙基和戊二烯基阳离子。虽然带有形式正电荷的位置的烷基化作用稳定了这些阳离子也就不足为奇了,但其他几种作用却不太明显。首先,不带正电荷的离域阳离子位置的烷基化是有益的,其程度约为带电荷位置的四分之一至三分之一。第二,接受质子的位置的烷基化不利于质子化。最后,至少在非循环系统中,两端对称为3°,另一端为1°的较不对称的模式适度偏向于两端为2°的较对称的取代模式。



























 京公网安备 11010802027423号
京公网安备 11010802027423号