当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Transfer Hydrogenation of Alkenes Using Ethanol Catalyzed by A NCP Pincer Iridium Complex: Scope and Mechanism
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-08 , DOI: 10.1021/jacs.8b01038 Yulei Wang 1 , Zhidao Huang 1 , Xuebing Leng 1 , Huping Zhu 1 , Guixia Liu 1 , Zheng Huang 1
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-08 , DOI: 10.1021/jacs.8b01038 Yulei Wang 1 , Zhidao Huang 1 , Xuebing Leng 1 , Huping Zhu 1 , Guixia Liu 1 , Zheng Huang 1
Affiliation
|
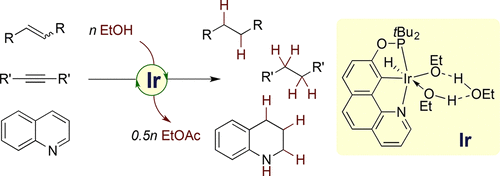
The first general catalytic approach to effecting transfer hydrogenation (TH) of unactivated alkenes using ethanol as the hydrogen source is described. A new NCP-type pincer iridium complex (BQ-NCOP)IrHCl containing a rigid benzoquinoline backbone has been developed for efficient, mild TH of unactivated C-C multiple bonds with ethanol, forming ethyl acetate as the sole byproduct. A wide variety of alkenes, including multisubstituted alkyl alkenes, aryl alkenes, and heteroatom-substituted alkenes, as well as O- or N-containing heteroarenes and internal alkynes, are suitable substrates. Importantly, the (BQ-NCOP)Ir/EtOH system exhibits high chemoselectivity for alkene hydrogenation in the presence of reactive functional groups, such as ketones and carboxylic acids. Furthermore, the reaction with C2D5OD provides a convenient route to deuterium-labeled compounds. Detailed kinetic and mechanistic studies have revealed that monosubstituted alkenes (e.g., 1-octene, styrene) and multisubstituted alkenes (e.g., cyclooctene (COE)) exhibit fundamental mechanistic difference. The OH group of ethanol displays a normal kinetic isotope effect (KIE) in the reaction of styrene, but a substantial inverse KIE in the case of COE. The catalysis of styrene or 1-octene with relatively strong binding affinity to the Ir(I) center has (BQ-NCOP)IrI(alkene) adduct as an off-cycle catalyst resting state, and the rate law shows a positive order in EtOH, inverse first-order in styrene, and first-order in the catalyst. In contrast, the catalysis of COE has an off-cycle catalyst resting state of (BQ-NCOP)IrIII(H)[O(Et)···HO(Et)···HOEt] that features a six-membered iridacycle consisting of two hydrogen-bonds between one EtO ligand and two EtOH molecules, one of which is coordinated to the Ir(III) center. The rate law shows a negative order in EtOH, zeroth-order in COE, and first-order in the catalyst. The observed inverse KIE corresponds to an inverse equilibrium isotope effect for the pre-equilibrium formation of (BQ-NCOP)IrIII(H)(OEt) from the catalyst resting state via ethanol dissociation. Regardless of the substrate, ethanol dehydrogenation is the slow segment of the catalytic cycle, while alkene hydrogenation occurs readily following the rate-determining step, that is, β-hydride elimination of (BQ-NCOP)Ir(H)(OEt) to form (BQ-NCOP)Ir(H)2 and acetaldehyde. The latter is effectively converted to innocent ethyl acetate under the catalytic conditions, thus avoiding the catalyst poisoning via iridium-mediated decarbonylation of acetaldehyde.
中文翻译:

NCP钳形铱配合物催化乙醇转移氢化烯烃:范围和机制
描述了使用乙醇作为氢源实现未活化烯烃的转移氢化 (TH) 的第一种通用催化方法。已经开发出一种新的 NCP 型钳形铱络合物 (BQ-NCOP)IrHCl,其含有刚性苯并喹啉主链,可有效、温和地将未活化的 CC 多键与乙醇进行 TH,形成乙酸乙酯作为唯一的副产品。各种烯烃,包括多取代的烷基烯烃、芳基烯烃和杂原子取代的烯烃,以及含 O 或 N 的杂芳烃和内部炔烃,都是合适的底物。重要的是,(BQ-NCOP)Ir/EtOH 系统在反应性官能团(如酮和羧酸)存在下对烯烃氢化表现出高化学选择性。此外,与 C2D5OD 的反应为氘标记化合物提供了一条便捷的途径。详细的动力学和机理研究表明,单取代烯烃(如 1-辛烯、苯乙烯)和多取代烯烃(如环辛烯 (COE))表现出基本的机理差异。乙醇的 OH 基团在苯乙烯反应中显示出正常的动力学同位素效应 (KIE),但在 COE 的情况下显示出显着的反向 KIE。对Ir(I)中心具有较强结合亲和力的苯乙烯或1-辛烯的催化具有(BQ-NCOP)IrI(烯烃)加合物作为非循环催化剂静止状态,速率规律在EtOH中呈正序,在苯乙烯中为逆一级,在催化剂中为一级。相比之下,COE 的催化具有 (BQ-NCOP)IrIII(H)[O(Et)...HO(Et)...HOEt] 的非循环催化剂静止状态,具有由两个氢组成的六元环- 一个 EtO 配体和两个 EtOH 分子之间的键,其中一个与 Ir(III) 中心配位。速率定律在 EtOH 中显示为负序,在 COE 中为零级,在催化剂中为一级。观察到的逆 KIE 对应于从催化剂静止状态通过乙醇解离 (BQ-NCOP)IrIII(H)(OEt) 的预平衡形成的逆平衡同位素效应。无论底物如何,乙醇脱氢是催化循环的缓慢部分,而烯烃加氢很容易在速率决定步骤之后发生,即 (BQ-NCOP)Ir(H)(OEt) 的 β-氢化物消除形成(BQ-NCOP)Ir(H)2 和乙醛。
更新日期:2018-03-08
中文翻译:

NCP钳形铱配合物催化乙醇转移氢化烯烃:范围和机制
描述了使用乙醇作为氢源实现未活化烯烃的转移氢化 (TH) 的第一种通用催化方法。已经开发出一种新的 NCP 型钳形铱络合物 (BQ-NCOP)IrHCl,其含有刚性苯并喹啉主链,可有效、温和地将未活化的 CC 多键与乙醇进行 TH,形成乙酸乙酯作为唯一的副产品。各种烯烃,包括多取代的烷基烯烃、芳基烯烃和杂原子取代的烯烃,以及含 O 或 N 的杂芳烃和内部炔烃,都是合适的底物。重要的是,(BQ-NCOP)Ir/EtOH 系统在反应性官能团(如酮和羧酸)存在下对烯烃氢化表现出高化学选择性。此外,与 C2D5OD 的反应为氘标记化合物提供了一条便捷的途径。详细的动力学和机理研究表明,单取代烯烃(如 1-辛烯、苯乙烯)和多取代烯烃(如环辛烯 (COE))表现出基本的机理差异。乙醇的 OH 基团在苯乙烯反应中显示出正常的动力学同位素效应 (KIE),但在 COE 的情况下显示出显着的反向 KIE。对Ir(I)中心具有较强结合亲和力的苯乙烯或1-辛烯的催化具有(BQ-NCOP)IrI(烯烃)加合物作为非循环催化剂静止状态,速率规律在EtOH中呈正序,在苯乙烯中为逆一级,在催化剂中为一级。相比之下,COE 的催化具有 (BQ-NCOP)IrIII(H)[O(Et)...HO(Et)...HOEt] 的非循环催化剂静止状态,具有由两个氢组成的六元环- 一个 EtO 配体和两个 EtOH 分子之间的键,其中一个与 Ir(III) 中心配位。速率定律在 EtOH 中显示为负序,在 COE 中为零级,在催化剂中为一级。观察到的逆 KIE 对应于从催化剂静止状态通过乙醇解离 (BQ-NCOP)IrIII(H)(OEt) 的预平衡形成的逆平衡同位素效应。无论底物如何,乙醇脱氢是催化循环的缓慢部分,而烯烃加氢很容易在速率决定步骤之后发生,即 (BQ-NCOP)Ir(H)(OEt) 的 β-氢化物消除形成(BQ-NCOP)Ir(H)2 和乙醛。


























 京公网安备 11010802027423号
京公网安备 11010802027423号