Bioorganic & Medicinal Chemistry ( IF 3.5 ) Pub Date : 2018-03-09 , DOI: 10.1016/j.bmc.2018.03.009 Somaye Ghasemy , Júlia García-Pindado , Fatemeh Aboutalebi , Kianoush Dormiani , Meritxell Teixidó , Morteza Malakoutikhah
|
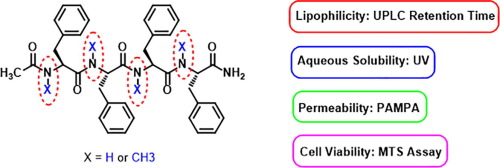
N-methylation is a powerful method to modify the physicochemical properties of peptides. We previously found that a fully N-methylated tetrapeptide, Ac-(N-MePhe)4-CONH2, was more lipophilic than its non-methylated analog Ac-(Phe)4-CONH2. In addition, the former crossed artificial and cell membranes while the latter did not. Here we sought to optimize the physicochemical properties of peptides and address how the number and position of N-methylated amino acids affect these properties. To this end, 15 analogs of Ac-(Phe)4-CONH2 were designed and synthesized in solid-phase. The solubility of the peptides in water and their lipophilicity, as measured by ultra performance liquid chromatography (UPLC) retention times, were determined. To study the permeability of the peptides, the Parallel Artificial Membrane Permeability Assay (PAMPA) was used as an in vitro model of the blood–brain barrier (BBB). Contrary to the parent peptide, the 15 analogs crossed the artificial membrane, thereby showing that N-methylation improved permeability. We also found that N-methylation enhanced lipophilicity but decreased the water solubility of peptides. Our results showed that both the number and position of N-methylated residues are important factors governing the physicochemical properties of peptides. There was no correlation between the number of N-methylated amide bonds and any of the properties measured. However, for the peptides consecutively N-methylated from the N-terminus to the C-terminus (p1, p5, p11, p12 and p16), lipophilicity correlated well with the number of N-methylated amide bonds and the permeability of the peptides. Moreover, the peptides were non-toxic to HEK293T cells, as determined by the 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay.
中文翻译:

调节基于肽的血脑屏障穿梭蛋白的理化特性
N-甲基化是修饰肽的理化性质的有力方法。我们先前发现,完全N-甲基化的四肽Ac-(N- MePhe)4 -CONH 2比其非甲基化的类似物Ac-(Phe)4 -CONH 2更具亲脂性。另外,前者穿过人造膜和细胞膜,而后者则没有。在这里,我们试图优化肽的物理化学性质,并解决N-甲基化氨基酸的数量和位置如何影响这些性质。为此,Ac-(Phe)4 -CONH 2的15个类似物设计并固相合成。通过超高效液相色谱(UPLC)保留时间测定,确定了肽在水中的溶解度和亲脂性。为了研究这些肽的通透性,使用平行人工膜通透性测定(PAMPA)作为血脑屏障(BBB)的体外模型。与亲本肽相反,这15个类似物穿过了人工膜,从而表明N-甲基化提高了通透性。我们还发现,N-甲基化增强了亲脂性,但降低了肽的水溶性。我们的结果表明N的数量和位置-甲基化残基是控制肽的物理化学性质的重要因素。N-甲基化酰胺键的数目与所测得的任何性质之间都没有相关性。然而,对于从N末端到C末端连续N-甲基化的肽(p1,p5,p11,p12和p16),亲脂性与N-甲基化酰胺键的数目和肽的渗透性具有良好的相关性。此外,根据3-(4,5-二甲基噻唑-2-基)-5-(3-羧基甲氧基苯基)-2-(4-磺基苯基)-2H-四唑鎓( MTS)分析。



























 京公网安备 11010802027423号
京公网安备 11010802027423号