Journal of the American Society for Mass Spectrometry ( IF 3.2 ) Pub Date : 2018-03-08 , DOI: 10.1007/s13361-018-1903-4 Yuejie Zhao 1 , Jeong Yeh Yang 2 , David F. Thieker 2 , Yongmei Xu 3 , Chengli Zong 2 , Geert-Jan Boons 2 , Jian Liu 3 , Robert J. Woods 2 , Kelley W. Moremen 2 , I. Jonathan Amster 1
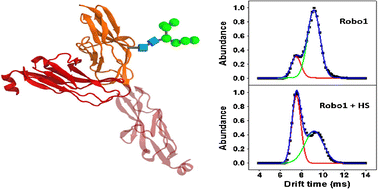
Roundabout 1 (Robo1) interacts with its receptor Slit to regulate axon guidance, axon branching, and dendritic development in the nervous system and to regulate morphogenesis and many cell functions in the nonneuronal tissues. This interaction is known to be critically regulated by heparan sulfate (HS). Previous studies suggest that HS is required to promote the binding of Robo1 to Slit to form the minimal signaling complex, but the molecular details and the structural requirements of HS for this interaction are still unclear. Here, we describe the application of traveling wave ion mobility spectrometry (TWIMS) to study the conformational details of the Robo1-HS interaction. The results suggest that Robo1 exists in two conformations that differ by their compactness and capability to interact with HS. The results also suggest that the highly flexible interdomain hinge region connecting the Ig1 and Ig2 domains of Robo1 plays an important functional role in promoting the Robo1-Slit interaction. Moreover, variations in the sulfation pattern and size of HS were found to affect its binding affinity and selectivity to interact with different conformations of Robo1. Both MS measurements and CIU experiments show that the Robo1-HS interaction requires the presence of a specific size and pattern of modification of HS. Furthermore, the effect of N-glycosylation on the conformation of Robo1 and its binding modes with HS is reported.
ᅟ
中文翻译:

Robo1-乙酰肝素硫酸盐相互作用的行波离子迁移谱(TWIMS)研究
回旋处1(Robo1)与受体Slit相互作用,调节神经系统中的轴突导向,轴突分支和树突状发育,并调节非神经组织中的形态发生和许多细胞功能。已知这种相互作用受到硫酸乙酰肝素(HS)的严格调节。先前的研究表明需要HS来促进Robo1与Slit的结合以形成最小的信号复合物,但是该相互作用的HS分子细节和结构要求仍然不清楚。在这里,我们描述了行波离子迁移谱(TWIMS)的应用,以研究Robo1-HS相互作用的构象细节。结果表明,Robo1存在两种构象,两者的紧凑性和与HS相互作用的能力不同。结果还表明,连接Robo1的Ig1和Ig2结构域的高柔性域间铰链区在促进Robo1-Slit相互作用中起着重要的功能作用。此外,发现硫酸化模式和HS大小的变化会影响其结合亲和力和与Robo1不同构象相互作用的选择性。MS测量和CIU实验均表明Robo1-HS相互作用需要HS修饰的特定大小和模式。此外,效果 发现硫酸盐的硫酸化模式和大小的变化会影响其结合亲和力以及与不同构型的Robo1相互作用的选择性。MS测量和CIU实验均表明Robo1-HS相互作用需要HS修饰的特定大小和模式。此外,效果 发现硫酸盐的硫酸化模式和大小的变化会影响其结合亲和力以及与不同构型的Robo1相互作用的选择性。MS测量和CIU实验均表明Robo1-HS相互作用需要HS修饰的特定大小和模式。此外,效果报道了Robo1的构象及其与HS的结合模式的N-糖基化。

ᅟ



























 京公网安备 11010802027423号
京公网安备 11010802027423号