Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-03-07 , DOI: 10.1016/j.actbio.2018.02.019 Yejun Hu , Jisheng Ran , Zefeng Zheng , Zhangchu Jin , Xiao Chen , Zi Yin , Chenqi Tang , Yangwu Chen , Jiayun Huang , Huihui Le , Ruijian Yan , Ting Zhu , Junjuan Wang , Junxin Lin , Kan Xu , Yiting Zhou , Wei Zhang , Youzhi Cai , Pioletti Dominique , Boon Chin Heng , Weishan Chen , Weiliang Shen , Hong-Wei Ouyang
|
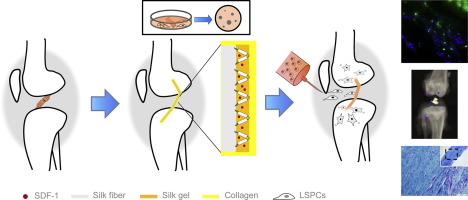
Anterior cruciate ligament (ACL) is one of the most difficult tissues to heal once injured. Ligament regeneration and tendon-bone junction healing are two major goals of ACL reconstruction. This study aimed to investigate the synergistic therapeutic effects of Stromal cell-derived factor 1(SDF-1)-releasing collagen-silk (CSF) scaffold combined with intra-articular injection of ligament-derived stem/progenitor cells (LSPCs) for ACL regeneration and the amelioration in the long-term complication of osteoarthritis (OA). The stem cell recruitment ability of CSF scaffold and the multipotency, particularly the tendon forming ability of LSPCs from rabbits were characterized in vitro, while the synergistic effect of the CSF scaffold and LSPCs for ACL regeneration and OA amelioration were investigated in vivo at 1, 3, and 6 months with a rabbit ACL reconstruction model. The CSF scaffold was used as a substitute for the ACL, and LSPCs were injected into the joint cavity after 7 days of the ACL reconstruction. CSF scaffold displayed a controlled release pattern for the encapsulated protein for up to 7 days with an increased stiffness in the mechanical property. LSPCs, which exhibited highly I Collagen and CXCR4 expression, were attracted by SDF-1 and successfully relocated into the CSF scaffold at 1 month in vivo. At 3 and 6 months post-treatment, the CSF scaffold combined with LSPCs (CSFL group) enhanced the regeneration of ACL tissue, and promoted bone tunnel healing. Furthermore, the OA progression was impeded efficiently. Our findings here provided a new strategy that using stem cell recruiting CSF scaffold with tissue-specific stem cells, could be a promising solution for ACL regeneration.
Significance
In this study, we developed a silk scaffold with increased stiffness and SDF-1 controlled release capacity for ligament repair. This advanced scaffold transplantation combined with intra-articular injection of LSPCs (which was isolated from rabbit ligament for the first time in this study) promoted the regeneration of both the tendinous and bone tunnel portion of ACL. This therapeutic strategy also ameliorated cartilage degeneration and reduced the severity of arthrofibrosis. Hence, combining LSPCs injection with SDF-1-releasing silk scaffold is demonstrated as a therapeutic strategy for ACL regeneration and OA treatment in the clinic.
中文翻译:

外源基质衍生因子-1释放丝支架结合关节内注射祖细胞促进骨韧带骨再生。
前交叉韧带(ACL)是受伤后最难愈合的组织之一。韧带再生和腱-骨连接愈合是ACL重建的两个主要目标。这项研究旨在探讨基质细胞源性因子1(SDF-1)释放胶原蛋白丝(CSF)支架联合关节内注射韧带来源的干/祖细胞(LSPCs)对ACL再生的协同治疗作用并改善骨关节炎(OA)的长期并发症。CSF支架和多潜能性,尤其是肌腱形成从兔LSPCs的能力进行了表征的体外干细胞募集的能力,而CSF支架和LSPCs为ACL再生和OA改善的协同效果进行了研究体内在1、3和6个月时使用兔子ACL重建模型。ASF重建7天后,将CSF支架用作ACL的替代物,并在关节腔中注入LSPC。CSF支架在长达7天的时间内表现出了被包封蛋白质的控制释放模式,并且机械性能的刚性提高了。LSPC高度表达I胶原和CXCR4表达,被SDF-1吸引,并在1个月的体内成功转移到CSF支架中。在治疗后3和6个月,CSF支架与LSPCs联合使用(CSFL组)增强了ACL组织的再生,并促进了骨隧道的愈合。此外,OA进展被有效地阻碍。我们的发现提供了一种新策略,即使用具有组织特异性干细胞的干细胞募集脑脊液支架,可能是ACL再生的有希望的解决方案。
意义
在这项研究中,我们开发了一种具有增强刚度和SDF-1控释能力的丝支架,用于韧带修复。这种先进的支架移植与关节内注射LSPCs(在本研究中首次从兔韧带中分离)相结合,促进了ACL的肌腱和骨隧道部分的再生。这种治疗策略还改善了软骨变性并降低了关节炎的严重程度。因此,在临床上,将LSPCs注射与SDF-1释放丝支架相结合被证明是ACL再生和OA治疗的治疗策略。



























 京公网安备 11010802027423号
京公网安备 11010802027423号