Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-03-07 , DOI: 10.1016/j.bioorg.2018.02.020 Hayat Ullah , Fazal Rahim , Muhammad Taha , Imad Uddin , Abdul Wadood , Syed Adnan Ali Shah , Rai Khalid Farooq , Mohsan Nawaz , Zainul Wahab , Khalid Mohammed Khan
|
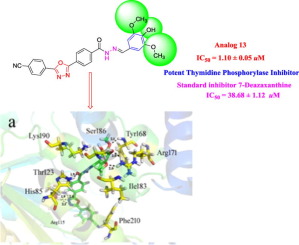
We have synthesized oxadiazole derivatives (1–16), characterized by 1H NMR, 13C NMR and HREI-MS and screened for thymidine phosphorylase inhibitory potential. All derivatives display varied degree of thymidine phosphorylase inhibition in the range of 1.10 ± 0.05 to 49.60 ± 1.30 μM when compared with the standard inhibitor 7-Deazaxanthine having an IC50 value 38.68 ± 1.12 μM. Structure activity relationships (SAR) has been established for all compounds to explore the role of substitution and nature of functional group attached to the phenyl ring which applies imperious effect on thymidine phosphorylase activity. Molecular docking study was performed to understand the binding interaction of the most active derivatives with enzyme active site.
中文翻译:

恶二唑衍生物的合成,分子对接研究及体外胸苷磷酸化酶抑制潜力
我们合成了恶二唑衍生物(1 – 16),其特征在于1 H NMR,13 C NMR和HREI-MS,并筛选了胸苷磷酸化酶抑制潜能。与具有IC 50的标准抑制剂7-Deazaxanthine相比,所有衍生物均显示出对胸苷磷酸化酶的抑制程度在1.10±0.05至49.60±1.30μM范围内。值38.68±1.12μM。已经建立了所有化合物的结构活性关系(SAR),以研究取代作用和与苯环相连的官能团的性质,该作用对胸苷磷酸化酶活性具有重要作用。进行了分子对接研究以了解最具活性的衍生物与酶活性位点的结合相互作用。


























 京公网安备 11010802027423号
京公网安备 11010802027423号