当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Free energy calculations and molecular properties of substrate translocation through OccAB porins†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1039/c7cp08299a Dehbia Benkerrou 1, 2, 3, 4 , Matteo Ceccarelli 1, 2, 3, 4
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1039/c7cp08299a Dehbia Benkerrou 1, 2, 3, 4 , Matteo Ceccarelli 1, 2, 3, 4
Affiliation
|
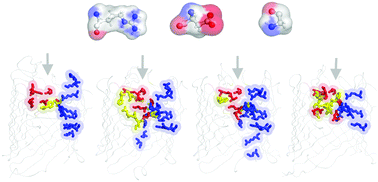
One of the greatest health threats facing modern medicine is the emergence of new bacterial strains which are increasingly resistant to almost all currently available antibiotics. According to a CDC (Center for Disease Control and Prevention) report published in 2013, 63% of Acinetobacter species have been identified as Multidrug resistant strains. As for other Gram-negative bacteria, the presence of an outer membrane increases the intrinsic resistance of A. baumannii to most antibiotics. The outer membrane of A. baumannii possesses several specific porins that control the selectivity for different polar substrates in a way that is still poorly understood. Recently, the X-ray crystal structures of 4 related porins, termed OccAB1–4, were solved at high resolution, providing a framework to study the structural and functional characteristics of these porins in filtering natural substrates. Here, we first use molecular dynamics simulations on OccAB proteins to investigate the stability and dynamics of the pores, and to establish their common biophysical features. We then applied metadynamics simulations to evaluate the free energy costs required for polar substrates to overcome the pore. Together, the comparative analysis of the OccAB porins not only sheds light on how these channels could function as potential antibiotic gateways, but also allows identification of putative affinity sites that represent a common path through which other molecules can transit.
中文翻译:

通过OccAB孔蛋白进行底物易位的自由能计算和分子特性†
现代医学面临的最大的健康威胁之一是新细菌菌株的出现,这些菌株对几乎所有当前可用的抗生素具有越来越大的抵抗力。根据CDC(疾病控制与预防中心)在2013年发布的报告,已确认63%的不动杆菌属多药耐药菌株。至于其他革兰氏阴性细菌,外膜的存在会增加鲍曼不动杆菌对大多数抗生素的内在抗性。鲍曼不动杆菌的外膜拥有几种特定的孔蛋白,这些孔蛋白以一种尚不为人所知的方式控制着不同极性底物的选择性。最近,以高分辨率解决了4个相关孔蛋白的X射线晶体结构,称为OccAB1-4的问题,为研究这些孔蛋白在过滤天然底物中的结构和功能特性提供了框架。在这里,我们首先使用OccAB蛋白的分子动力学模拟来研究孔的稳定性和动力学,并建立它们共同的生物物理特征。然后,我们应用了元动力学模拟来评估极性基材克服孔隙所需的自由能成本。总之,对OccAB孔蛋白的比较分析不仅揭示了这些通道如何充当潜在的抗生素通道,
更新日期:2018-03-07
中文翻译:

通过OccAB孔蛋白进行底物易位的自由能计算和分子特性†
现代医学面临的最大的健康威胁之一是新细菌菌株的出现,这些菌株对几乎所有当前可用的抗生素具有越来越大的抵抗力。根据CDC(疾病控制与预防中心)在2013年发布的报告,已确认63%的不动杆菌属多药耐药菌株。至于其他革兰氏阴性细菌,外膜的存在会增加鲍曼不动杆菌对大多数抗生素的内在抗性。鲍曼不动杆菌的外膜拥有几种特定的孔蛋白,这些孔蛋白以一种尚不为人所知的方式控制着不同极性底物的选择性。最近,以高分辨率解决了4个相关孔蛋白的X射线晶体结构,称为OccAB1-4的问题,为研究这些孔蛋白在过滤天然底物中的结构和功能特性提供了框架。在这里,我们首先使用OccAB蛋白的分子动力学模拟来研究孔的稳定性和动力学,并建立它们共同的生物物理特征。然后,我们应用了元动力学模拟来评估极性基材克服孔隙所需的自由能成本。总之,对OccAB孔蛋白的比较分析不仅揭示了这些通道如何充当潜在的抗生素通道,


























 京公网安备 11010802027423号
京公网安备 11010802027423号