Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Epigenetic reprogramming enables the transition from primordial germ cell to gonocyte
Nature ( IF 64.8 ) Pub Date : 2018-03-01 , DOI: 10.1038/nature25964 Peter W. S. Hill , Harry G. Leitch , Cristina E. Requena , Zhiyi Sun , Rachel Amouroux , Monica Roman-Trufero , Malgorzata Borkowska , Jolyon Terragni , Romualdas Vaisvila , Sarah Linnett , Hakan Bagci , Gopuraja Dharmalingham , Vanja Haberle , Boris Lenhard , Yu Zheng , Sriharsa Pradhan , Petra Hajkova
Nature ( IF 64.8 ) Pub Date : 2018-03-01 , DOI: 10.1038/nature25964 Peter W. S. Hill , Harry G. Leitch , Cristina E. Requena , Zhiyi Sun , Rachel Amouroux , Monica Roman-Trufero , Malgorzata Borkowska , Jolyon Terragni , Romualdas Vaisvila , Sarah Linnett , Hakan Bagci , Gopuraja Dharmalingham , Vanja Haberle , Boris Lenhard , Yu Zheng , Sriharsa Pradhan , Petra Hajkova
|
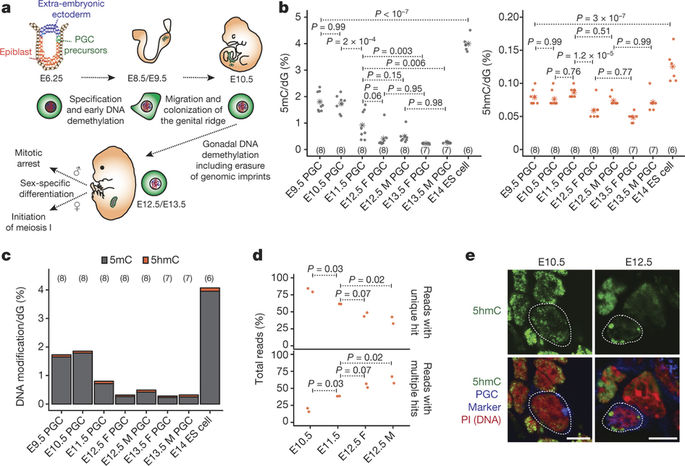
Gametes are highly specialized cells that can give rise to the next generation through their ability to generate a totipotent zygote. In mice, germ cells are first specified in the developing embryo around embryonic day (E) 6.25 as primordial germ cells (PGCs). Following subsequent migration into the developing gonad, PGCs undergo a wave of extensive epigenetic reprogramming around E10.5-E11.5, including genome-wide loss of 5-methylcytosine. The underlying molecular mechanisms of this process have remained unclear, leading to our inability to recapitulate this step of germline development in vitro. Here we show, using an integrative approach, that this complex reprogramming process involves coordinated interplay among promoter sequence characteristics, DNA (de)methylation, the polycomb (PRC1) complex and both DNA demethylation-dependent and -independent functions of TET1 to enable the activation of a critical set of germline reprogramming-responsive genes involved in gamete generation and meiosis. Our results also reveal an unexpected role for TET1 in maintaining but not driving DNA demethylation in gonadal PGCs. Collectively, our work uncovers a fundamental biological role for gonadal germline reprogramming and identifies the epigenetic principles of the PGC-to-gonocyte transition that will help to guide attempts to recapitulate complete gametogenesis in vitro.
中文翻译:

表观遗传重编程使原始生殖细胞转变为生殖细胞
配子是高度特化的细胞,可以通过它们产生全能受精卵的能力产生下一代。在小鼠中,生殖细胞首先在胚胎日 (E) 6.25 左右的发育胚胎中被指定为原始生殖细胞 (PGC)。在随后迁移到发育中的性腺后,PGC 在 E10.5-E11.5 周围经历了一波广泛的表观遗传重编程,包括全基因组 5-甲基胞嘧啶的丢失。该过程的潜在分子机制仍不清楚,导致我们无法在体外重现生殖系发育的这一步骤。在这里,我们使用综合方法表明,这种复杂的重编程过程涉及启动子序列特征、DNA(去)甲基化、多梳 (PRC1) 复合物以及 TET1 的 DNA 去甲基化依赖性和非甲基化功能,能够激活一组关键的生殖系重编程响应基因,这些基因涉及配子生成和减数分裂。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。
更新日期:2018-03-01
中文翻译:

表观遗传重编程使原始生殖细胞转变为生殖细胞
配子是高度特化的细胞,可以通过它们产生全能受精卵的能力产生下一代。在小鼠中,生殖细胞首先在胚胎日 (E) 6.25 左右的发育胚胎中被指定为原始生殖细胞 (PGC)。在随后迁移到发育中的性腺后,PGC 在 E10.5-E11.5 周围经历了一波广泛的表观遗传重编程,包括全基因组 5-甲基胞嘧啶的丢失。该过程的潜在分子机制仍不清楚,导致我们无法在体外重现生殖系发育的这一步骤。在这里,我们使用综合方法表明,这种复杂的重编程过程涉及启动子序列特征、DNA(去)甲基化、多梳 (PRC1) 复合物以及 TET1 的 DNA 去甲基化依赖性和非甲基化功能,能够激活一组关键的生殖系重编程响应基因,这些基因涉及配子生成和减数分裂。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。我们的结果还揭示了 TET1 在维持但不驱动性腺 PGC 中 DNA 去甲基化方面的意外作用。总的来说,我们的工作揭示了性腺种系重编程的基本生物学作用,并确定了 PGC 到生殖细胞转变的表观遗传原理,这将有助于指导在体外重现完整配子发生的尝试。



























 京公网安备 11010802027423号
京公网安备 11010802027423号