当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
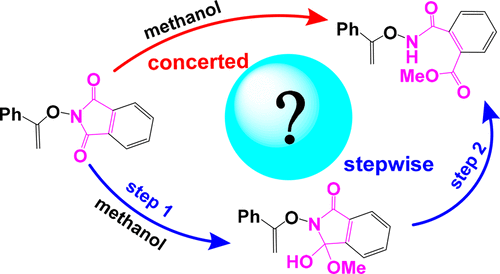
Methanol-Assisted Phthalimide Ring Opening: Concerted or Stepwise Mechanism?
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.7b11347 Wei-Hao Chen 1 , Xuejiao J. Gao 1 , Xingfa Gao 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.7b11347 Wei-Hao Chen 1 , Xuejiao J. Gao 1 , Xingfa Gao 1
Affiliation
|
The opening of the five-membered ring is the essential step for phthalimide and its derivatives to be used as the reactants in many chemical synthetic routes. Reportedly, such ring opening follows the concerted mechanism in methanol solvent, which, however, has an unreasonably high energy barrier (36.3 kcal mol–1 at the M06-2X/6-311++G(d,p) level of theory). By density functional theory calculations, we report that this ring opening prefers the alternatively stepwise mechanism. The stepwise mechanism has a much lower energy barrier (21.0 kcal mol–1 at the same level of theory) and thus is much more completive than the concerted one. The stepwise mechanism should be considered as the dominant mechanism responsible for the phthalimide ring opening when studying the kinetics of the relevant synthetic reactions in the future.
中文翻译:

甲醇辅助邻苯二甲酰亚胺开环:协同还是逐步的机制?
五元环的打开是邻苯二甲酰亚胺及其衍生物在许多化学合成路线中用作反应物的必不可少的步骤。据报道,这种开环遵循了在甲醇溶剂中的协同机理,然而,该机理具有不合理的高能垒(在理论水平为M06-2X / 6-311 ++ G(d,p)时为36.3 kcal mol –1) 。通过密度泛函理论计算,我们报告此开环更喜欢交替的逐步机制。步进机制的能垒低得多(21.0 kcal mol –1在相同的理论水平上),因此比一致的方法更具竞争力。将来研究相关合成反应的动力学时,应将逐步机理视为负责邻苯二甲酰亚胺开环的主要机理。
更新日期:2018-03-07
中文翻译:

甲醇辅助邻苯二甲酰亚胺开环:协同还是逐步的机制?
五元环的打开是邻苯二甲酰亚胺及其衍生物在许多化学合成路线中用作反应物的必不可少的步骤。据报道,这种开环遵循了在甲醇溶剂中的协同机理,然而,该机理具有不合理的高能垒(在理论水平为M06-2X / 6-311 ++ G(d,p)时为36.3 kcal mol –1) 。通过密度泛函理论计算,我们报告此开环更喜欢交替的逐步机制。步进机制的能垒低得多(21.0 kcal mol –1在相同的理论水平上),因此比一致的方法更具竞争力。将来研究相关合成反应的动力学时,应将逐步机理视为负责邻苯二甲酰亚胺开环的主要机理。



























 京公网安备 11010802027423号
京公网安备 11010802027423号