当前位置:
X-MOL 学术
›
J. Phys. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Reaction F + C2H4: Rate Constant and Yields of the Reaction Products as a Function of Temperature over 298–950 K
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.8b01371 Yuri Bedjanian 1
The Journal of Physical Chemistry A ( IF 2.9 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acs.jpca.8b01371 Yuri Bedjanian 1
Affiliation
|
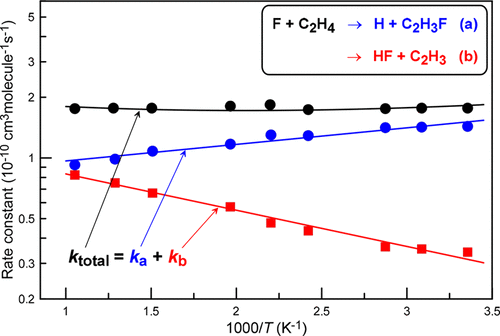
The kinetics and products of the reaction of F + C2H4 have been studied in a discharge flow reactor combined with an electron impact ionization mass spectrometer at nearly 2 Torr total pressure of helium in the temperature range 298–950 K. The total rate constant of the reaction, k1 = (1.78 ± 0.30) × 10–10 cm3 molecule–1 s–1, determined under pseudo-first-order conditions, monitoring the kinetics of F atom consumption in excess of C2H4, was found to be temperature independent in the temperature range used. H, C2H3F, and HF were identified as the reaction products. Absolute measurements of the yields of these species allowed to determine the branching ratios, k1b/k1 = (0.73 ± 0.07) exp(−(425 ± 45)/T) and k1a/k1 = 1 – (0.73 ± 0.07) exp(−(425 ± 45)/T) and partial rate constants for addition–elimination (H + C2H3F) and H atom abstraction (HF + C2H3) pathways of the title reaction: k1a = (0.80 ± 0.07) × 10–10exp(189 ± 37/T) and k1b = (1.26 ± 0.13) × 10–10exp(−414 ± 45/T) cm3 molecule–1 s–1, respectively, at T = 298–950 K and with 2σ quoted uncertainties. The overall reaction rate constant can be adequately described by both the temperature independent value and as a sum of k1a and k1b. The kinetic and mechanistic data from the present study are discussed in comparison with previous absolute and relative measurements and theoretical calculations.
中文翻译:

F + C 2 H 4反应:298–950 K上反应速率常数和反应产物的产率与温度的关系
F + C 2 H 4的反应动力学和产物已在放电流反应器中结合电子碰撞电离质谱仪在298–950 K的氦气总压力下以近2 Torr的总压力进行了研究。总速率反应常数k 1 =(1.78±0.30)×10 –10 cm 3分子–1 s –1,在拟一级条件下测定,监测超过C 2 H 4的F原子消耗的动力学,发现在所使用的温度范围内,温度与温度无关。高,低2高3F和HF被确定为反应产物。绝对测量这些物种的产量可以确定分支比,k 1b / k 1 =(0.73±0.07)exp(-(425±45)/ T)和k 1a / k 1 = 1-(0.73±0.07) )exp(-(425±45)/ T)以及标题反应的加成消除(H + C 2 H 3 F)和H原子抽象(HF + C 2 H 3)路径的部分速率常数:k 1a = (0.80±0.07)×10 –10 exp(189±37 / T)和k1b =(1.26±0.13)×10 –10 exp(-414±45 / T)cm 3分子–1 s –1,分别在T = 298–950 K和2σ引用的不确定性下。总反应速率常数既可以由温度无关的值来表示,也可以由k 1a和k 1b的总和来适当描述。与先前的绝对和相对测量值以及理论计算结果进行了比较,讨论了本研究的动力学和力学数据。
更新日期:2018-03-07
中文翻译:

F + C 2 H 4反应:298–950 K上反应速率常数和反应产物的产率与温度的关系
F + C 2 H 4的反应动力学和产物已在放电流反应器中结合电子碰撞电离质谱仪在298–950 K的氦气总压力下以近2 Torr的总压力进行了研究。总速率反应常数k 1 =(1.78±0.30)×10 –10 cm 3分子–1 s –1,在拟一级条件下测定,监测超过C 2 H 4的F原子消耗的动力学,发现在所使用的温度范围内,温度与温度无关。高,低2高3F和HF被确定为反应产物。绝对测量这些物种的产量可以确定分支比,k 1b / k 1 =(0.73±0.07)exp(-(425±45)/ T)和k 1a / k 1 = 1-(0.73±0.07) )exp(-(425±45)/ T)以及标题反应的加成消除(H + C 2 H 3 F)和H原子抽象(HF + C 2 H 3)路径的部分速率常数:k 1a = (0.80±0.07)×10 –10 exp(189±37 / T)和k1b =(1.26±0.13)×10 –10 exp(-414±45 / T)cm 3分子–1 s –1,分别在T = 298–950 K和2σ引用的不确定性下。总反应速率常数既可以由温度无关的值来表示,也可以由k 1a和k 1b的总和来适当描述。与先前的绝对和相对测量值以及理论计算结果进行了比较,讨论了本研究的动力学和力学数据。



























 京公网安备 11010802027423号
京公网安备 11010802027423号