当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
pH-Dependent Catalytic Reaction Pathway for Water Splitting at the BiVO4–Water Interface from the Band Alignment
ACS Energy Letters ( IF 22.0 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsenergylett.8b00104 Francesco Ambrosio 1 , Julia Wiktor 1 , Alfredo Pasquarello 1
ACS Energy Letters ( IF 22.0 ) Pub Date : 2018-03-07 00:00:00 , DOI: 10.1021/acsenergylett.8b00104 Francesco Ambrosio 1 , Julia Wiktor 1 , Alfredo Pasquarello 1
Affiliation
|
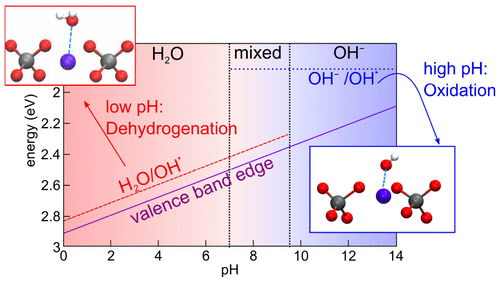
We align the band edges of BiVO4 at the interface with liquid water by combining advanced electronic-structure calculations, molecular dynamics simulations, and a computational hydrogen electrode. After accounting for spin–orbit coupling and thermal and nuclear quantum motions, we achieve good agreement with experiment, particularly with one-shot GW calculations and semiempirically tuned hybrid functionals. The pH-dependent mechanism of the water oxidation reaction is discussed in consideration of the pH at the point of zero charge, the pKa of adsorbed water molecules, and the redox levels of the rate-determining step of the reaction. The mechanism pertaining to acidic conditions is found to dominate over a large pH range. The kinetically more favorable oxidation of hydroxyl ions is favored only in highly alkaline conditions and could be hampered by corrosion processes. Advanced electronic-structure methods are shown to be instrumental in overcoming the erroneous physical picture achieved at the semilocal level of theory.
中文翻译:

pH依赖的催化反应途径,通过能带对准在BiVO 4-水界面处进行水分解
通过结合先进的电子结构计算,分子动力学模拟和计算氢电极,将BiVO 4的能带边缘与液态水对齐。在考虑了自旋-轨道耦合以及热和核量子运动之后,我们与实验取得了良好的一致性,尤其是单次GW计算和半经验调谐的混合泛函。考虑到零电荷点的pH p K a,讨论了水氧化反应的pH依赖性机理。吸附的水分子的组成,以及反应速率确定步骤的氧化还原水平。发现与酸性条件有关的机理在较大的pH范围内占主导。仅在高度碱性条件下才有利于羟基离子的动力学上更有利的氧化,并且可能会受到腐蚀过程的阻碍。先进的电子结构方法被证明有助于克服在半局部理论水平上获得的错误的物理图像。
更新日期:2018-03-07
中文翻译:

pH依赖的催化反应途径,通过能带对准在BiVO 4-水界面处进行水分解
通过结合先进的电子结构计算,分子动力学模拟和计算氢电极,将BiVO 4的能带边缘与液态水对齐。在考虑了自旋-轨道耦合以及热和核量子运动之后,我们与实验取得了良好的一致性,尤其是单次GW计算和半经验调谐的混合泛函。考虑到零电荷点的pH p K a,讨论了水氧化反应的pH依赖性机理。吸附的水分子的组成,以及反应速率确定步骤的氧化还原水平。发现与酸性条件有关的机理在较大的pH范围内占主导。仅在高度碱性条件下才有利于羟基离子的动力学上更有利的氧化,并且可能会受到腐蚀过程的阻碍。先进的电子结构方法被证明有助于克服在半局部理论水平上获得的错误的物理图像。



























 京公网安备 11010802027423号
京公网安备 11010802027423号