Chemical Physics Letters ( IF 2.8 ) Pub Date : 2018-03-07 , DOI: 10.1016/j.cplett.2018.03.007 Iwona Anusiewicz , Piotr Skurski
|
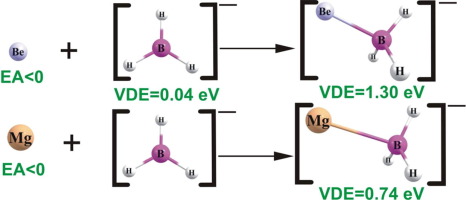
Stability of BeBH3 and MgBH3 molecules and their (BeBH3)– and (MgBH3)– anions is investigated on the basis of correlated ab initio calculations. The electronic and thermodynamic stability of all species is confirmed by estimating the excess electron binding energies of the anions and by evaluating the Gibbs free energies for various fragmentation paths. The bonding effects in BeBH3 and MgBH3 have been identified as the result of alkaline earth metal ns2 lone-pair donation to the empty 2p boron orbital. Adiabatic and vertical electronic stabilities of the (BeBH3)– and (MgBH3)– anions were found to span 1.114–1.301 and 0.675–0.744 eV range, respectively.
中文翻译:

将Be或Mg附着于BH 3导致形成能够形成稳定阴离子的BeBH 3和MgBH 3分子
基于相关的从头算计算,研究了BeBH 3和MgBH 3分子及其(BeBH 3)-和(MgBH 3)-阴离子的稳定性。通过估计阴离子的过量电子结合能并通过评估各种断裂路径的吉布斯自由能,可以确认所有物质的电子和热力学稳定性。已将BeBH 3和MgBH 3中的键合效应确定为碱土金属n s 2孤对向空的2 p硼轨道供体的结果。(BeBH的绝热和垂直电子稳定性3)-和(MgBH 3)- ,发现阴离子跨越1.114-1.301和0.675-0.744电子伏特范围内,分别。


























 京公网安备 11010802027423号
京公网安备 11010802027423号