当前位置:
X-MOL 学术
›
Bioconjugate Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Arginine-Rich Cell-Penetrating Peptides Require Nucleolin and Cholesterol-Poor Subdomains for Translocation across Membranes
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00805 Annely Lorents 1, 2 , Pille Säälik 1, 3 , Ülo Langel 2, 4 , Margus Pooga 1, 2
Bioconjugate Chemistry ( IF 4.7 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.bioconjchem.7b00805 Annely Lorents 1, 2 , Pille Säälik 1, 3 , Ülo Langel 2, 4 , Margus Pooga 1, 2
Affiliation
|
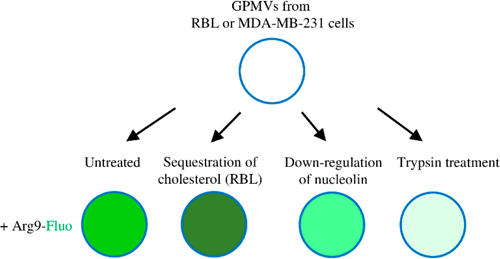
Proficient transport vectors called cell-penetrating peptides (CPPs) internalize into eukaryotic cells mostly via endocytic pathways and facilitate the uptake of various cargo molecules attached to them. However, some CPPs are able to induce disturbances in the plasma membrane and translocate through it seemingly in an energy-independent manner. For understanding this phenomenon, giant plasma membrane vesicles (GPMVs) derived from the cells are a beneficial model system, since GPMVs have a complex membrane composition comparable to the cells yet lack cellular energy-dependent mechanisms. We investigated the translocation of arginine-rich CPPs into GPMVs with different membrane compositions. Our results demonstrate that lower cholesterol content favors accumulation of nona-arginine and, additionally, sequestration of cholesterol increases the uptake of the CPPs in vesicles with higher cholesterol packing density. Furthermore, the proteins on the surface of vesicles are essential for the uptake of arginine-rich CPPs: downregulation of nucleolin decreases the accumulation and digestion of proteins on the membrane suppresses translocation even more efficiently.
中文翻译:

富含精氨酸的细胞穿透肽需要Nucleolin和胆固醇低的子域才能跨膜转运。
称为细胞穿透肽(CPPs)的高效转运载体主要通过内吞途径进入真核细胞,并促进摄取附着在其上的各种货物分子。但是,某些CPP似乎能够以与能量无关的方式在质膜上引起干扰并通过其移位。为了理解该现象,源自细胞的巨大质膜囊泡(GPMV)是有益的模型系统,因为GPMV具有与细胞相当的复杂膜组成,但缺乏细胞能量依赖性机制。我们调查了富含精氨酸的CPPs向具有不同膜组成的GPMVs的易位性。我们的结果表明,较低的胆固醇含量有利于壬精氨酸的积累,此外,胆固醇的隔离增加了胆固醇堆积密度较高的囊泡中CPP的吸收。此外,囊泡表面的蛋白质对于摄取富含精氨酸的CPP是必不可少的:核仁素的下调减少了膜上的蛋白质的积累和消化,从而更有效地抑制了转运。
更新日期:2018-03-06
中文翻译:

富含精氨酸的细胞穿透肽需要Nucleolin和胆固醇低的子域才能跨膜转运。
称为细胞穿透肽(CPPs)的高效转运载体主要通过内吞途径进入真核细胞,并促进摄取附着在其上的各种货物分子。但是,某些CPP似乎能够以与能量无关的方式在质膜上引起干扰并通过其移位。为了理解该现象,源自细胞的巨大质膜囊泡(GPMV)是有益的模型系统,因为GPMV具有与细胞相当的复杂膜组成,但缺乏细胞能量依赖性机制。我们调查了富含精氨酸的CPPs向具有不同膜组成的GPMVs的易位性。我们的结果表明,较低的胆固醇含量有利于壬精氨酸的积累,此外,胆固醇的隔离增加了胆固醇堆积密度较高的囊泡中CPP的吸收。此外,囊泡表面的蛋白质对于摄取富含精氨酸的CPP是必不可少的:核仁素的下调减少了膜上的蛋白质的积累和消化,从而更有效地抑制了转运。



























 京公网安备 11010802027423号
京公网安备 11010802027423号