Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Manipulating Single Microdroplets of NaCl Solutions: Solvent Dissolution, Microcrystallization, and Crystal Morphology
Langmuir ( IF 3.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.langmuir.7b03977 Anders Utoft 1 , Koji Kinoshita 1 , Deborah L. Bitterfield 2 , David Needham 1, 3, 4
Langmuir ( IF 3.9 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.langmuir.7b03977 Anders Utoft 1 , Koji Kinoshita 1 , Deborah L. Bitterfield 2 , David Needham 1, 3, 4
Affiliation
|
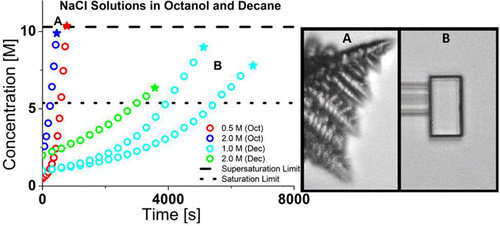
A new “three-micropipette manipulation technique” for forming, dehydrating, crystallizing, and resolvating nanograms of salt material has been developed to study supersaturated single microdroplets and microcrystals. This is the first report of studies that have measured in situ both supersaturation (as homogeneous nucleation) and saturation (as microcrystal redissolution) for single microdroplets of NaCl solution using the micropipette technique. This work reports a measure of the critical supersaturation concentration for homogeneous nucleation of NaCl (10.3 ± 0.3 M) at a supersaturation fraction of S = 1.9, the saturation concentration of NaCl in aqueous solution as measured with nanograms of material (5.5 ± 0.1 M), the diffusion coefficient for water in octanol, D = (1.96 ± 0.10) × 10–6 cm2/s, and the effect of the solvent’s activity on dissolution kinetics. It is further shown that the same Epstein–Plesset (EP) model, which was originally developed for diffusion-controlled dissolution and uptake of gas, and successfully applied to liquid-in-liquid dissolution, can now also be applied to describe the diffusion-controlled uptake of water from a water-saturated environment using the extended activity-based model of Bitterfield et al. This aspect of the EP model has not previously been tested using single microdroplets. Finally, it is also reported how the water dissolution rate, rate of NaCl concentration change, resulting crystal structure, and the time frame of initial crystal growth are affected by changing the bathing medium from octanol to decane. A much slower loss of water-solvent and concomitant slower up-concentration of the NaCl solute resulted in a lower tendency to nucleate and slower crystal growth because much less excess material was available at the onset of nucleation in the decane system as compared to the octanol system. Thus, the crystal structure is reported to be dendritic for NaCl solution microdroplets dissolving rapidly and nucleating violently in octanol, while they are formed as single cubic crystals in a gentler way for solution-dissolution in decane. These new techniques and analyses can now also be used for any other system where all relevant parameters are known. An example of this is control of drug/hydrogel/emulsion particle size change due to solvent uptake.
中文翻译:

操纵NaCl溶液的单个微滴:溶剂溶解,微晶化和晶体形态
已经开发出一种用于形成,脱水,结晶和溶解纳克盐材料的新的“三种微量移液器操作技术”,以研究过饱和的单个微滴和微晶。这是第一份研究报告,该报告使用微量移液器技术对NaCl溶液的单个微滴原位测量了过饱和度(均质成核)和饱和度(微晶再溶解)。这项工作报告了在S = 1.9的过饱和分数下,NaCl的均匀成核(10.3±0.3 M)的临界过饱和浓度的度量,该浓度是用纳克材料(5.5±0.1 M)测量的水溶液中的NaCl饱和浓度,水在辛醇中的扩散系数,D=(1.96±0.10)×10 –6 cm 2/ s,以及溶剂活性对溶解动力学的影响。进一步证明,相同的爱泼斯坦-普莱塞(EP)模型最初是为扩散控制的气体溶解和吸收而开发的,并成功地应用于液-液溶解,现在也可以用于描述扩散- Bitterfield等人的扩展基于活动的模型控制了从饱和水环境中吸收水。EP模型的这一方面以前尚未使用单个微滴进行过测试。最后,还报道了将浴液从辛醇改为癸烷会如何影响水的溶解速率,NaCl浓度变化速率,所得晶体结构以及初始晶体生长的时间框架。与癸醇相比,癸烷系统中成核开始时可用的过量材料少得多,因此,水溶剂的损失要慢得多,同时伴随NaCl溶质的缓慢上升,导致成核的趋势降低,晶体的生长也较慢。系统。因此,据报道,NaCl溶液的微滴在辛醇中迅速溶解并剧烈成核,而晶体结构是树枝状的,而它们以较温和的方式形成单立方晶体,从而可以在癸烷中溶解。这些新技术和分析现在也可以用于所有相关参数已知的任何其他系统。这方面的一个例子是控制由于溶剂吸收而引起的药物/水凝胶/乳液粒径的变化。
更新日期:2018-03-06
中文翻译:

操纵NaCl溶液的单个微滴:溶剂溶解,微晶化和晶体形态
已经开发出一种用于形成,脱水,结晶和溶解纳克盐材料的新的“三种微量移液器操作技术”,以研究过饱和的单个微滴和微晶。这是第一份研究报告,该报告使用微量移液器技术对NaCl溶液的单个微滴原位测量了过饱和度(均质成核)和饱和度(微晶再溶解)。这项工作报告了在S = 1.9的过饱和分数下,NaCl的均匀成核(10.3±0.3 M)的临界过饱和浓度的度量,该浓度是用纳克材料(5.5±0.1 M)测量的水溶液中的NaCl饱和浓度,水在辛醇中的扩散系数,D=(1.96±0.10)×10 –6 cm 2/ s,以及溶剂活性对溶解动力学的影响。进一步证明,相同的爱泼斯坦-普莱塞(EP)模型最初是为扩散控制的气体溶解和吸收而开发的,并成功地应用于液-液溶解,现在也可以用于描述扩散- Bitterfield等人的扩展基于活动的模型控制了从饱和水环境中吸收水。EP模型的这一方面以前尚未使用单个微滴进行过测试。最后,还报道了将浴液从辛醇改为癸烷会如何影响水的溶解速率,NaCl浓度变化速率,所得晶体结构以及初始晶体生长的时间框架。与癸醇相比,癸烷系统中成核开始时可用的过量材料少得多,因此,水溶剂的损失要慢得多,同时伴随NaCl溶质的缓慢上升,导致成核的趋势降低,晶体的生长也较慢。系统。因此,据报道,NaCl溶液的微滴在辛醇中迅速溶解并剧烈成核,而晶体结构是树枝状的,而它们以较温和的方式形成单立方晶体,从而可以在癸烷中溶解。这些新技术和分析现在也可以用于所有相关参数已知的任何其他系统。这方面的一个例子是控制由于溶剂吸收而引起的药物/水凝胶/乳液粒径的变化。



























 京公网安备 11010802027423号
京公网安备 11010802027423号