当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Discovery of [cis-3-({(5R)-5-[(7-Fluoro-1,1-dimethyl-2,3-dihydro-1H-inden-5-yl)carbamoyl]-2-methoxy-7,8-dihydro-1,6-naphthyridin-6(5H)-yl}carbonyl)cyclobutyl]acetic Acid (TAK-828F) as a Potent, Selective, and Orally Available Novel Retinoic Acid Receptor-Related Orphan Receptor γt Inverse Agonist
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00061 Mitsunori Kono 1 , Atsuko Ochida 1 , Tsuneo Oda 1 , Takashi Imada 1 , Yoshihiro Banno 1 , Naohiro Taya 1 , Shinichi Masada 1 , Tetsuji Kawamoto 1 , Kazuko Yonemori 1 , Yoshi Nara 1 , Yoshiyuki Fukase 1 , Tomoya Yukawa 1 , Hidekazu Tokuhara 1 , Robert Skene 2 , Bi-Ching Sang 2 , Isaac D. Hoffman 2 , Gyorgy P. Snell 2 , Keiko Uga 1 , Akira Shibata 1 , Keiko Igaki 1 , Yoshiki Nakamura 1 , Hideyuki Nakagawa 1 , Noboru Tsuchimori 1 , Masashi Yamasaki 1 , Junya Shirai 1 , Satoshi Yamamoto 1
Journal of Medicinal Chemistry ( IF 7.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jmedchem.8b00061 Mitsunori Kono 1 , Atsuko Ochida 1 , Tsuneo Oda 1 , Takashi Imada 1 , Yoshihiro Banno 1 , Naohiro Taya 1 , Shinichi Masada 1 , Tetsuji Kawamoto 1 , Kazuko Yonemori 1 , Yoshi Nara 1 , Yoshiyuki Fukase 1 , Tomoya Yukawa 1 , Hidekazu Tokuhara 1 , Robert Skene 2 , Bi-Ching Sang 2 , Isaac D. Hoffman 2 , Gyorgy P. Snell 2 , Keiko Uga 1 , Akira Shibata 1 , Keiko Igaki 1 , Yoshiki Nakamura 1 , Hideyuki Nakagawa 1 , Noboru Tsuchimori 1 , Masashi Yamasaki 1 , Junya Shirai 1 , Satoshi Yamamoto 1
Affiliation
|
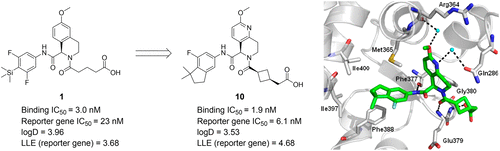
A series of tetrahydronaphthyridine derivatives as novel RORγt inverse agonists were designed and synthesized. We reduced the lipophilicity of tetrahydroisoquinoline compound 1 by replacement of the trimethylsilyl group and SBDD-guided scaffold exchange, which successfully afforded compound 7 with a lower log D value and tolerable in vitro activity. Consideration of LLE values in the subsequent optimization of the carboxylate tether led to the discovery of [cis-3-({(5R)-5-[(7-fluoro-1,1-dimethyl-2,3-dihydro-1H-inden-5-yl)carbamoyl]-2-methoxy-7,8-dihydro-1,6-naphthyridin-6(5H)-yl}carbonyl)cyclobutyl]acetic acid, TAK-828F (10), which showed potent RORγt inverse agonistic activity, excellent selectivity against other ROR isoforms and nuclear receptors, and a good pharmacokinetic profile. In animal studies, oral administration of compound 10 exhibited robust and dose-dependent inhibition of IL-17A cytokine expression in a mouse IL23-induced gene expression assay. Furthermore, development of clinical symptoms in a mouse experimental autoimmune encephalomyelitis model was significantly reduced. Compound 10 was selected as a clinical compound for the treatment of Th17-driven autoimmune diseases.
中文翻译:

[[顺式-3-({(5 R)-5-[(7-氟-1,1-二甲基-2,3-二氢-1 H-茚满-5-基)氨基甲酰基] -2-甲氧基-]的发现7,8-二氢-1,6-萘啶-6(5 H)-基}羰基)环丁基]乙酸(TAK-828F)作为有效,选择性和口服可用的新型视黄酸受体相关孤儿受体γt逆激动剂
设计并合成了一系列新型RORγt反向激动剂四氢萘啶衍生物。我们通过替换三甲基甲硅烷基和SBDD引导的支架交换来降低四氢异喹啉化合物1的亲脂性,这成功地提供了具有较低log D值和可耐受的体外活性的化合物7。在随后优化羧酸酯系链中考虑LLE值导致发现[顺式-3-({(5 R)-5-[(7-氟-1,1-二甲基-2,3-二氢-1 H-茚满-5-基)氨基甲酰基] -2-甲氧基-7,8-二氢-1,6-萘吡啶-6(5 H)-基}羰基)环丁基]乙酸,TAK-828F(10),它具有强大的RORγt反向激动活性,对其他ROR亚型和核受体的优异选择性以及良好的药代动力学特征。在动物研究中,化合物10的口服给药在小鼠IL23诱导的基因表达分析中显示出对IL-17A细胞因子表达的强烈和剂量依赖性抑制。此外,在小鼠实验性自身免疫性脑脊髓炎模型中临床症状的发展显着减少。选择化合物10作为用于治疗Th17驱动的自身免疫疾病的临床化合物。
更新日期:2018-03-06
中文翻译:

[[顺式-3-({(5 R)-5-[(7-氟-1,1-二甲基-2,3-二氢-1 H-茚满-5-基)氨基甲酰基] -2-甲氧基-]的发现7,8-二氢-1,6-萘啶-6(5 H)-基}羰基)环丁基]乙酸(TAK-828F)作为有效,选择性和口服可用的新型视黄酸受体相关孤儿受体γt逆激动剂
设计并合成了一系列新型RORγt反向激动剂四氢萘啶衍生物。我们通过替换三甲基甲硅烷基和SBDD引导的支架交换来降低四氢异喹啉化合物1的亲脂性,这成功地提供了具有较低log D值和可耐受的体外活性的化合物7。在随后优化羧酸酯系链中考虑LLE值导致发现[顺式-3-({(5 R)-5-[(7-氟-1,1-二甲基-2,3-二氢-1 H-茚满-5-基)氨基甲酰基] -2-甲氧基-7,8-二氢-1,6-萘吡啶-6(5 H)-基}羰基)环丁基]乙酸,TAK-828F(10),它具有强大的RORγt反向激动活性,对其他ROR亚型和核受体的优异选择性以及良好的药代动力学特征。在动物研究中,化合物10的口服给药在小鼠IL23诱导的基因表达分析中显示出对IL-17A细胞因子表达的强烈和剂量依赖性抑制。此外,在小鼠实验性自身免疫性脑脊髓炎模型中临床症状的发展显着减少。选择化合物10作为用于治疗Th17驱动的自身免疫疾病的临床化合物。



























 京公网安备 11010802027423号
京公网安备 11010802027423号