当前位置:
X-MOL 学术
›
Metallomics
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multimodal LA-ICP-MS and nanoSIMS imaging enables copper mapping within photoreceptor megamitochondria in a zebrafish model of Menkes disease
Metallomics ( IF 3.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7mt00349h Cheri M. Ackerman 1, 2, 3, 4 , Peter K. Weber 4, 5, 6, 7 , Tong Xiao 1, 2, 3, 4, 8 , Bao Thai 1, 2, 3, 4 , Tiffani J. Kuo 1, 2, 3, 4 , Emily Zhang 1, 2, 3, 4 , Jennifer Pett-Ridge 4, 5, 6, 7 , Christopher J. Chang 1, 2, 3, 4, 8
Metallomics ( IF 3.4 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7mt00349h Cheri M. Ackerman 1, 2, 3, 4 , Peter K. Weber 4, 5, 6, 7 , Tong Xiao 1, 2, 3, 4, 8 , Bao Thai 1, 2, 3, 4 , Tiffani J. Kuo 1, 2, 3, 4 , Emily Zhang 1, 2, 3, 4 , Jennifer Pett-Ridge 4, 5, 6, 7 , Christopher J. Chang 1, 2, 3, 4, 8
Affiliation
|
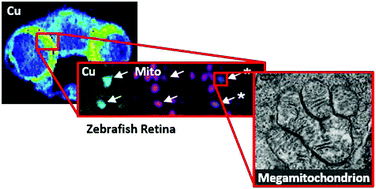
Copper is essential for eukaryotic life, and animals must acquire this nutrient through the diet and distribute it to cells and organelles for proper function of biological targets. Indeed, mutations in the central copper exporter ATP7A contribute to a spectrum of diseases, including Menkes disease, with symptoms ranging from neurodegeneration to lax connective tissue. As such, a better understanding of the fundamental impacts of ATP7A mutations on in vivo copper distributions is of relevance to those affected by these diseases. Here we combine metal imaging and optical imaging techniques at a variety of spatial resolutions to identify tissues and structures with altered copper levels in the Calamitygw71 zebrafish model of Menkes disease. Rapid profiling of tissue slices with LA-ICP-MS identified reduced copper levels in the brain, neuroretina, and liver of Menkes fish compared to control specimens. High resolution nanoSIMS imaging of the neuroretina, combined with electron and confocal microscopies, identified the megamitochondria of photoreceptors as loci of copper accumulation in wildtype fish, with lower levels of megamitochondrial copper observed in Calamitygw71 zebrafish. Interestingly, this localized copper decrease does not result in impaired photoreceptor development or altered megamitochondrial morphology, suggesting the prioritization of copper at sufficient levels for maintaining essential mitochondrial functions. Together, these data establish the Calamitygw71 zebrafish as an optically transparent in vivo model for the study of neural copper misregulation, illuminate a role for the ATP7A copper exporter in trafficking copper to the neuroretina, and highlight the utility of combining multiple imaging techniques for studying metals in whole organism settings with spatial resolution.
中文翻译:

多峰LA-ICP-MS和nanoSIMS成像使Menkes病斑马鱼模型中的感光器巨大线粒体内的铜定位成为可能
铜对于真核生物是必不可少的,动物必须通过饮食获得这种营养,并将其分配到细胞和细胞器中,以实现生物学目标的正常功能。实际上,中央铜出口者ATP7A的突变会导致一系列疾病,包括Menkes疾病,其症状从神经变性到结缔组织松弛不等。因此,更好地了解ATP7A突变对体内铜分布的基本影响与那些受这些疾病影响的人有关。在这里,我们结合了各种空间分辨率的金属成像和光学成像技术,以识别Calamity gw71中铜含量发生变化的组织和结构斑马鱼病的斑马鱼模型。使用LA-ICP-MS对组织切片进行快速分析,发现与对照标本相比,Menkes鱼的大脑,神经视网膜和肝脏中的铜含量降低。神经视网膜的高分辨率nanoSIMS成像,结合电子显微镜和共聚焦显微镜,将感光体的线粒体鉴定为野生型鱼中铜积累的场所,而在灾难性gw71斑马鱼中观察到的线粒体铜水平较低。有趣的是,这种局部的铜减少不会导致光感受器发育受损或巨线粒体形态发生改变,这表明铜的优先次序应足以维持必需的线粒体功能。这些数据共同构成了灾难gw71斑马鱼作为一种光学透明的体内模型,用于研究神经铜的失调,阐明了ATP7A铜出口者在将铜运输到神经视网膜中的作用,并着重指出了将多种成像技术结合起来用于研究整个生物体环境中的金属和空间的实用性解析度。
更新日期:2018-03-21
中文翻译:

多峰LA-ICP-MS和nanoSIMS成像使Menkes病斑马鱼模型中的感光器巨大线粒体内的铜定位成为可能
铜对于真核生物是必不可少的,动物必须通过饮食获得这种营养,并将其分配到细胞和细胞器中,以实现生物学目标的正常功能。实际上,中央铜出口者ATP7A的突变会导致一系列疾病,包括Menkes疾病,其症状从神经变性到结缔组织松弛不等。因此,更好地了解ATP7A突变对体内铜分布的基本影响与那些受这些疾病影响的人有关。在这里,我们结合了各种空间分辨率的金属成像和光学成像技术,以识别Calamity gw71中铜含量发生变化的组织和结构斑马鱼病的斑马鱼模型。使用LA-ICP-MS对组织切片进行快速分析,发现与对照标本相比,Menkes鱼的大脑,神经视网膜和肝脏中的铜含量降低。神经视网膜的高分辨率nanoSIMS成像,结合电子显微镜和共聚焦显微镜,将感光体的线粒体鉴定为野生型鱼中铜积累的场所,而在灾难性gw71斑马鱼中观察到的线粒体铜水平较低。有趣的是,这种局部的铜减少不会导致光感受器发育受损或巨线粒体形态发生改变,这表明铜的优先次序应足以维持必需的线粒体功能。这些数据共同构成了灾难gw71斑马鱼作为一种光学透明的体内模型,用于研究神经铜的失调,阐明了ATP7A铜出口者在将铜运输到神经视网膜中的作用,并着重指出了将多种成像技术结合起来用于研究整个生物体环境中的金属和空间的实用性解析度。


























 京公网安备 11010802027423号
京公网安备 11010802027423号