Bioorganic Chemistry ( IF 5.1 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.bioorg.2018.02.023 Mohamed K.S. El-Nagar , Hajjaj H.M. Abdu-Allah , Ola I.A. Salem , Abdel-Hamid N. Kafafy , Hanan S.M. Farghaly
|
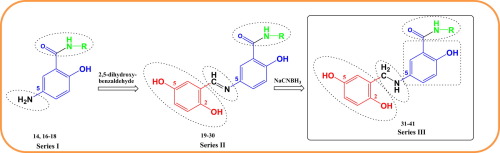
Three new series of 5-aminosalicylic acid derivatives; series I (14, 16–18), series II (19–30) and series III (31–41) were synthesized as potential dual COX-2/5-LOX inhibitors. Their chemical structures were confirmed using spectroscopic tools including IR, 1H NMR, 13C NMR, mass spectroscopy and elemental analyses. The anti-inflammatory activity for all target compounds was evaluated in vivo using carrageenan-induced paw edema. Compound 36 showed the highest anti-inflammatory activity (114.12%) relative to reference drug indomethacin at 4 h interval. Selected derivatives were evaluated in vitro to inhibit ovine COX-1, human recombinant COX-2 and 5-LOX enzymes. Compounds 34 & 35 exhibited significant COX-2 inhibition (IC50 = 0.10 µM) with significant COX-2 selectivity indices (SI = 135 & 145 respectively) approximate to celecoxib (IC50 = 0.049 µM, SI = 308.16) and exceeding indomethacin (IC50 = 0.51 µM, SI = 0.08). Interestingly, all compounds showed superior 5-LOX inhibitory activity about 2–5 times relative to zileuton. Compound 16 was the superlative 5-LOX inhibitor that revealed (IC50 = 3.41 µM) relative to zileuton (IC50 = 15.6 µM). Compounds 34, 35, 36 and 41 showed significant dual COX-2/5-LOX inhibitions. The gastric ulcerogenic effect of compound 36 was examined on gastric mucosa of albino rats and they showed superior GI safety profile compared with indomethacin. Molecular docking studies of the compounds into the binding sites of COX-1, COX-2 and 5-LOX allowed us to shed light on the binding mode of these novels dual COX and 5-LOX inhibitors.
中文翻译:

新型N-取代的5-氨基水杨酰胺作为环氧合酶和5-脂氧合酶的双重抑制剂:合成,生物学评估和对接研究
三个新的5-氨基水杨酸衍生物系列;系列I(14,16 - 18),系列II(19 - 30)和系列III(31 - 41)的合成作为潜在的双重COX-2/5-LOX抑制剂。使用包括IR,1 H NMR,13 C NMR,质谱和元素分析在内的光谱工具确认了它们的化学结构。使用角叉菜胶诱导的爪水肿在体内评估了所有目标化合物的抗炎活性。相对于参考药物消炎痛,化合物36显示出最高的抗炎活性(114.12%)每隔4小时 在体外评估了选定的衍生物,以抑制绵羊的COX-1,人重组COX-2和5-LOX酶。化合物34和35表现出显着的COX-2抑制作用(IC 50 = 0.10 µM),且具有显着的COX-2选择性指数(分别为SI = 135和145),接近塞来昔布(IC 50 = 0.049 µM,SI = 308.16),并超过消炎痛( IC 50 = 0.51 µM,SI = 0.08)。有趣的是,相对于齐留通,所有化合物均显示出优异的5-LOX抑制活性,约为zileuton的2至5倍。化合物16是显示出的最高级5-LOX抑制剂(IC 50 相对于齐留通(IC 50 = 15.6 µM)= 3.41 µM 。化合物34,35,36和41显示出显著双COX-2/5-LOX的抑制。研究了化合物36对白化病大鼠胃黏膜的胃溃疡作用,与吲哚美辛相比具有优越的胃肠道安全性。化合物对COX-1,COX-2和5-LOX结合位点的分子对接研究使我们得以阐明这些新型双重COX和5-LOX抑制剂的结合方式。


























 京公网安备 11010802027423号
京公网安备 11010802027423号