Journal of Controlled Release ( IF 10.8 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.jconrel.2018.03.006 Aditi Jhaveri , Pranali Deshpande , Bhushan Pattni , Vladimir Torchilin
|
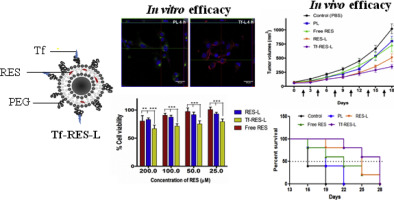
Glioblastomas (GBMs) are highly aggressive brain tumors with a very grim prognosis even after multi-modal therapeutic regimens. Conventional chemotherapeutic agents frequently lead to drug resistance and result in severe toxicities to non-cancerous tissues. Resveratrol (RES), a natural polyphenol with pleiotropic health benefits, has proven chemopreventive effects in all the stages of cancer including initiation, promotion and progression. However, the poor physico-chemical properties of RES severely limit its use as a free drug. In this study, RES was loaded into PEGylated liposomes (RES-L) to counter its drawbacks as a free drug. Since transferrin receptors (TfRs) are up-regulated in GBM, the liposome surface was modified with transferrin moieties (Tf-RES-L) to make them cancer cell-specific. The liposomal nanomedicines developed in this project were aimed at enhancing the physico-chemical properties of RES and exploiting the passive and active targeting capabilities of liposomes to effectively treat GBM.
The RES-L were stable, had a good drug-loading capacity, prolonged drug-release in vitro and were easily scalable. Flow cytometry and confocal microscopy were used to study the association with, and internalization of, Tf-L into U-87 MG cells. The Tf-RES-Ls were significantly more cytotoxic and induced higher levels of apoptosis accompanied by activation of caspases 3/7 in GBM cells when compared to free RES or RES-L. The ability of RES to arrest cells in the S-phase of the cell cycle, and selectively induce production of reactive oxygen species in cancer cells were probably responsible for its cytotoxic effects. The therapeutic efficacy of RES formulations was evaluated in a subcutaneous xenograft mouse model of GBM. A tumor growth inhibition study and a modified survival study showed that Tf-RES-Ls were more effective than other treatments in their ability to inhibit tumor growth and improve survival in mice. Overall, the liposomal nanomedicines of RES developed in this project exhibited favorable in vitro and in vivo efficacies, which warrant their further investigation for the treatment of GBMs.
中文翻译:

靶向转铁蛋白的负载白藜芦醇的脂质体用于治疗胶质母细胞瘤
胶质母细胞瘤(GBM)是高度侵袭性的脑肿瘤,即使采用多种模式的治疗方案,其预后也很差。常规化学治疗剂经常导致耐药性,并导致对非癌性组织的严重毒性。白藜芦醇(RES)是一种具有多效保健功能的天然多酚,已证明在癌症的所有阶段都具有化学预防作用,包括启动,促进和发展。但是,RES较差的理化性质严重限制了其作为游离药物的用途。在这项研究中,将RES装入聚乙二醇化脂质体(RES-L)中,以克服其作为游离药物的缺点。由于转铁蛋白受体(TfRs)在GBM中上调,因此脂质体表面被转铁蛋白部分(Tf-RES-L)修饰,使其具有癌细胞特异性。
RES-L稳定,具有良好的载药能力,体外药物释放时间长并且易于扩展。流式细胞术和共聚焦显微镜用于研究Tf-L与U-87 MG细胞的相关性和内在化。与游离RES或RES-L相比,Tf-RES-Ls具有更高的细胞毒性,并诱导更高水平的凋亡,并伴有半胱氨酸蛋白酶3/7在GBM细胞中的活化。RES在细胞周期的S期阻止细胞并选择性诱导癌细胞中活性氧生成的能力可能是其细胞毒性作用的原因。在GBM的皮下异种移植小鼠模型中评估了RES制剂的治疗功效。肿瘤生长抑制研究和改良的生存研究表明,Tf-RES-Ls在抑制肿瘤生长和改善小鼠生存能力方面比其他治疗更为有效。全面的,体外和体内疗效,值得进一步研究治疗GBM。



























 京公网安备 11010802027423号
京公网安备 11010802027423号