Journal of Hazardous Materials ( IF 13.6 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.jhazmat.2018.03.005 Neng-min Zhu , Yan-sheng Xu , Lichun Dai , Yun-fei Zhang , Guo-quan Hu
|
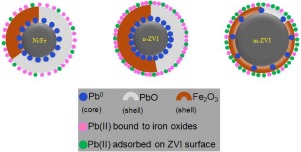
Zerovalent iron (ZVI) is an environmental-friendly reactive reagent for recovering heavy metals. However, the detailed recovery mechanism remains unclear due to a lack of quantitative analysis of recovery products. Herein, microscale ZVI, nanoscale ZVI and Ni/Fe nanoparticles were used to recover Pb(II) in aqueous solution and a sequential extraction procedure (SEP) was applied to determine the formed lead species quantitatively. At high initial Pb(II) concentration (500 mg L-1), more than 99.5% of Pb(II) was immobilized by Ni/Fe and n-ZVI, whereas m-ZVI caused inferior recovery efficiency (< 25%). XRD and XPS results revealed that Pb(II) was reduced to Pb0 prior to the formation of metal hydroxides as the external shell of ZVI. SEP results showed that the fraction bound to carbonates (PbO), fraction bound to iron oxides and exchangeable fraction were the main lead species conducted by Ni/Fe, n-ZVI and m-ZVI, respectively. Consequently, (co-)precipitation and specific adsorption dominated Pb(II) recovery by Ni/Fe and n-ZVI, whereas m-ZVI conducted Pb(II) recovery mainly via weak adsorption. The reactivity of ZVI toward Pb(II) followed the increasing order of m-ZVI << n-ZVI ≤ Ni/Fe. The detailed mechanisms of Pb(II) recovery conducted by different ZVI were proposed.
中文翻译:

顺序萃取分析在零价铁基颗粒回收Pb(II)中的应用
零价铁(ZVI)是一种用于回收重金属的环保反应试剂。但是,由于缺乏对回收产品的定量分析,详细的回收机制仍不清楚。在此,使用微米级ZVI,纳米级ZVI和Ni / Fe纳米颗粒回收水溶液中的Pb(II),并采用顺序萃取程序(SEP)定量确定形成的铅种类。在高的初始Pb(II)浓度(500 mg L -1)下,Ni / Fe和n-ZVI固定了超过99.5%的Pb(II),而m-ZVI导致回收率较低(<25%)。XRD和XPS结果表明Pb(II)还原为Pb 0在形成金属氢氧化物作为ZVI的外壳之前。SEP结果表明,与碳酸盐(PbO)结合的部分,与氧化铁结合的部分和可交换部分是分别由Ni / Fe,n-ZVI和m-ZVI进行的主要铅物种。因此,(共)沉淀和特定吸附主要是由Ni / Fe和n-ZVI回收Pb(II),而m-ZVI主要是通过弱吸附进行Pb(II)回收。ZVI对Pb(II)的反应性遵循m-ZVI << n-ZVI≤Ni / Fe的增加顺序。提出了不同ZVI进行Pb(II)回收的详细机理。


























 京公网安备 11010802027423号
京公网安备 11010802027423号