当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Optimum Number of Anchored Clathrate Water and Its Instantaneous Fluctuations Dictate Ice Plane Recognition Specificities of Insect Antifreeze Protein
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jpcb.8b00548 Sandipan Chakraborty 1 , Biman Jana 1
The Journal of Physical Chemistry B ( IF 3.3 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1021/acs.jpcb.8b00548 Sandipan Chakraborty 1 , Biman Jana 1
Affiliation
|
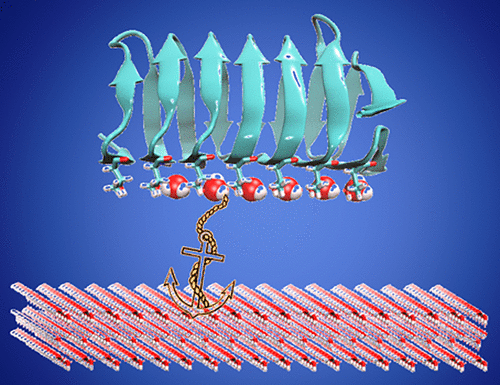
Ice recognition by antifreeze proteins (AFPs) is a subject of topical interest. Among several classes of AFPs, insect AFPs are hyperactive presumably due to their ability to adsorb on basal plane. However, the origin of the basal plane binding specificity is not clearly known. Present work aims to provide atomistic insight into the origin of basal plane recognition by an insect antifreeze protein. Free energy calculations reveal that the order of binding affinity of the AFP toward different ice planes is basal plane > prism plane > pyramidal plane. Critical insight reveals that the observed plane specificity is strongly correlated with the number and their instantaneous fluctuations of clathrate water forming hydrogen bonds with both ice binding surface (IBS) of AFP and ice surface, thus anchoring AFP to the ice surface. On basal plane, anchored clathrate water array is highly stable due to exact match in the periodicity of oxygen atom repeat distances of the ice surface and the threonine repeat distances at the IBS. The stability of anchored clathrate water array progressively decreases upon prism and pyramidal plane adsorption due to mismatch between the threonine ladder and oxygen atom repeat distance. Further analysis reveals that hydration around the methyl side-chains of threonine residues becomes highly significant at low temperature which stabilizes the anchored clathrate water array and dual hydrogen-bonding is a consequence of this stability. Structural insight gained from this study paves the way for rational designing of highly potent antifreeze-mimetic with potential industrial applications.
中文翻译:

固定防冻水的最佳数量及其瞬时起伏决定昆虫抗冻蛋白的冰平面识别特异性
防冻蛋白(AFP)对冰的识别已成为热门话题。在几类AFP中,昆虫AFP可能是高活性的,这是由于它们能够吸附在基面上。然而,基底平面结合特异性的起源尚不清楚。当前的工作旨在为昆虫抗冻蛋白对基础平面识别的起源提供原子学的见解。自由能计算表明,AFP对不同冰面的结合亲和力的顺序为基面>棱柱面>锥面。关键的洞察力表明,观察到的平面特异性与笼形水的数量及其瞬时波动密切相关,笼形水与AFP的冰结合表面(IBS)和冰表面形成氢键,从而将AFP锚定在冰表面。在基面上 由于冰表面的氧原子重复距离和IBS处的苏氨酸重复距离的周期性完全匹配,因此固定的笼形水阵列非常稳定。由于苏氨酸阶梯和氧原子重复距离之间的不匹配,锚固包合物水阵列的稳定性在棱镜和金字塔平面吸附时逐渐降低。进一步的分析表明,苏氨酸残基的甲基侧链周围的水合在低温下变得非常重要,这使锚定的包合物水阵列稳定,并且双重氢键是这种稳定性的结果。从这项研究中获得的结构洞察力为合理设计具有潜在工业应用潜力的高效防冻剂铺平了道路。
更新日期:2018-03-06
中文翻译:

固定防冻水的最佳数量及其瞬时起伏决定昆虫抗冻蛋白的冰平面识别特异性
防冻蛋白(AFP)对冰的识别已成为热门话题。在几类AFP中,昆虫AFP可能是高活性的,这是由于它们能够吸附在基面上。然而,基底平面结合特异性的起源尚不清楚。当前的工作旨在为昆虫抗冻蛋白对基础平面识别的起源提供原子学的见解。自由能计算表明,AFP对不同冰面的结合亲和力的顺序为基面>棱柱面>锥面。关键的洞察力表明,观察到的平面特异性与笼形水的数量及其瞬时波动密切相关,笼形水与AFP的冰结合表面(IBS)和冰表面形成氢键,从而将AFP锚定在冰表面。在基面上 由于冰表面的氧原子重复距离和IBS处的苏氨酸重复距离的周期性完全匹配,因此固定的笼形水阵列非常稳定。由于苏氨酸阶梯和氧原子重复距离之间的不匹配,锚固包合物水阵列的稳定性在棱镜和金字塔平面吸附时逐渐降低。进一步的分析表明,苏氨酸残基的甲基侧链周围的水合在低温下变得非常重要,这使锚定的包合物水阵列稳定,并且双重氢键是这种稳定性的结果。从这项研究中获得的结构洞察力为合理设计具有潜在工业应用潜力的高效防冻剂铺平了道路。


























 京公网安备 11010802027423号
京公网安备 11010802027423号