Journal of Controlled Release ( IF 10.8 ) Pub Date : 2018-03-06 , DOI: 10.1016/j.jconrel.2018.03.003 Quanying Bao , Bryan Newman , Yan Wang , Stephanie Choi , Diane J. Burgess
|
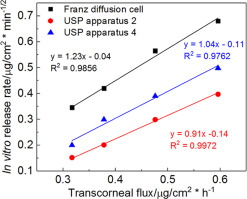
In vitro drug release testing and ex vivo transcorneal drug permeation can provide valuable information on the performance of the Q1/Q2 equivalent ointments prior to any animal studies. Good correlation between in vitro and ex vivo drug release may be indicative of good in vitro and in vivo correlation. Accordingly, it is important to investigate in vitro as well as ex vivo drug release from Q1/Q2 equivalent ophthalmic ointments and evaluate whether a correlation between these release profiles can be established. Four Q1/Q2 equivalent loteprednol etabonate ointments were prepared using different processing methods and excipient sources. The rheological parameters (crossover modulus and K value) of the four formulations were determined. The in vitro drug release testing of the four ointment formulations were performed using three different apparati (Franz diffusion cells, USP apparatus 2 with enhancer cells and USP apparatus 4 with semisolid adapters). Three models (zero order, logarithmic and the Higuchi model) were used to study the release kinetics of the ointment formulations. The transcorneal (rabbit corneas) permeation studies were performed using spherical joint Franz diffusion cells. The USP apparatus 4 method demonstrated better discriminatory ability compared to the USP apparatus 2 and the Franz diffusion cell methods. The in vitro release profiles of the four Q1/Q2 equivalent ointments with manufacturing differences showed a better fit using the Higuchi model (R2 > 0.98) for all three release testing methods, compared to the other two models. Ex vivo drug release through the rabbit corneas displayed zero order release kinetics. A logarithmic correlation between rheological parameters (crossover and K value) and transcorneal flux were established. In addition, a plot of the in vitro release rate against the ex vivo release flux of the four ointment formulations, yielded a straight line (R2 > 0.98) for all three release methods. Accordingly, the rheological parameters may be useful in predicting in vitro as well as ex vivo release properties.
中文翻译:

眼药膏中药物释放的体外和离体相关性
在进行任何动物研究之前,体外药物释放测试和离体透角膜药物渗透都可提供有关Q1 / Q2等效软膏性能的有价值的信息。良好的相关性在体外和体外药物释放可能表明良好的体外和体内的相关性。因此,重要的是要进行体外和离体研究从Q1 / Q2等效眼药膏中释放药物,并评估是否可以建立这些释放曲线之间的相关性。使用不同的加工方法和赋形剂来源制备了四种等效于Q1 / Q2的洛替泼诺依他博酯软膏。确定了四种配方的流变参数(交叉模量和K值)。在体外使用三种不同的设备(Franz扩散池,带增强剂池的USP装置2和带半固体接头的USP装置4)对四种软膏剂的药物释放测试。使用三种模型(零级,对数和Hi口模型)研究软膏剂型的释放动力学。使用球形关节Franz扩散池进行透角膜(兔角膜)渗透研究。与USP装置2和Franz扩散池方法相比,USP装置4的方法具有更好的区分能力。在体外与制造差异的四个Q1 / Q2等效软膏的释放曲线表明使用樋口模型(R更好的贴合2 与其他两个模型相比,所有三种发布测试方法均> 0.98)。通过兔角膜的离体药物释放显示零级释放动力学。建立了流变学参数(交叉和K值)与角膜通量之间的对数关系。另外,四种软膏制剂的体外释放速率相对于离体释放通量的图 对于所有三种释放方法均产生了一条直线(R 2 > 0.98)。因此,流变参数可用于预测体外以及离体释放特性。

























 京公网安备 11010802027423号
京公网安备 11010802027423号