当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Temperature-dependent phase behaviour of tetrahydrofuran–water alters solubilization of xylan to improve co-production of furfurals from lignocellulosic biomass
Green Chemistry ( IF 9.8 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7gc03608f Micholas Dean Smith 1, 2, 3, 4, 5 , Charles M. Cai 4, 6, 7, 8, 9 , Xiaolin Cheng 1, 2, 3, 4 , Loukas Petridis 1, 2, 3, 4, 5 , Jeremy C. Smith 1, 2, 3, 4, 5
Green Chemistry ( IF 9.8 ) Pub Date : 2018-03-06 00:00:00 , DOI: 10.1039/c7gc03608f Micholas Dean Smith 1, 2, 3, 4, 5 , Charles M. Cai 4, 6, 7, 8, 9 , Xiaolin Cheng 1, 2, 3, 4 , Loukas Petridis 1, 2, 3, 4, 5 , Jeremy C. Smith 1, 2, 3, 4, 5
Affiliation
|
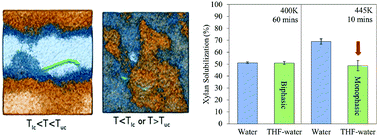
Xylan is an important polysaccharide found in the hemicellulose fraction of lignocellulosic biomass that can be hydrolysed to xylose and further dehydrated to the furfural, an important renewable platform fuel precursor. Here, pairing molecular simulation and experimental evidence, we reveal how the unique temperature-dependent phase behaviour of water–tetrahydrofuran (THF) co-solvent can delay xylan solubilization to synergistically improve catalytic co-processing of biomass to furfural and 5-HMF. Our results indicate, based on polymer correlations between polymer conformational behaviour and solvent quality, that both co-solvent and aqueous environments serve as ‘good’ solvents for xylan. Interestingly, the simulations also revealed that unlike other cell-wall components (i.e., lignin and cellulose), the make-up of the solvation shell of xylan in THF–water is dependent on the temperature-phase behaviour. At temperatures between 333 K and 418 K, THF and water become immiscible, and THF is evacuated from the solvation shell of xylan, while above and below this temperature range, THF and water are both present in the polysaccharide's solvation shell. This suggested that the solubilization of xylan in THF–water may be similar to aqueous-only solutions at temperatures between 333 K and 418 K and different outside this range. Experimental reactions on beachwood xylan corroborate this hypothesis by demonstrating 2-fold reduction of xylan solubilization in THF–water within a miscible temperature regime (445 K) and unchanged solubilization within an immiscible regime (400 K). Translating this phase-dependent behaviour to processing of maple wood chips, we demonstrate how the weaker xylan solvation in THF–water under miscible conditions can delay furfural production from xylan, allowing 5-HMF production from cellulose to “catch-up” such that their high yield production from biomass can be synergized in a single pot reaction.
中文翻译:

四氢呋喃-水的温度依赖性相行为改变了木聚糖的增溶作用,从而改善了木质纤维素生物质的糠醛联产
木聚糖是在木质纤维素生物质的半纤维素部分中发现的一种重要多糖,可以水解为木糖,然后进一步脱水为糠醛(一种重要的可再生平台燃料前体)。在这里,结合分子模拟和实验证据,我们揭示了水-四氢呋喃(THF)助溶剂独特的温度依赖性相行为如何可以延迟木聚糖增溶作用,从而协同改善生物质向糠醛和5-HMF的催化共处理。我们的结果表明,基于聚合物构象行为与溶剂质量之间的聚合物相关性,助溶剂和水性环境均可用作木聚糖的“良好”溶剂。有趣的是,模拟还显示出与其他细胞壁成分不同(即,木质素和纤维素),木聚糖在THF-水中的溶剂化壳的组成取决于温度相的行为。在333 K和418 K之间的温度下,THF和水变得不溶混,THF从木聚糖的溶剂化壳中抽出,而高于和低于此温度范围,THF和水都存在于多糖的溶剂化壳中。这表明在333 K和418 K之间的温度下,木聚糖在THF-水中的增溶作用可能类似于纯水溶液,但在此范围之外有所不同。通过证明在可混溶温度范围内(445 K)在THF水中的木聚糖溶解度降低2倍,而在不混溶范围内(400 K)的溶解度不变,对沙滩木木聚糖的实验反应证实了这一假设。
更新日期:2018-04-03
中文翻译:

四氢呋喃-水的温度依赖性相行为改变了木聚糖的增溶作用,从而改善了木质纤维素生物质的糠醛联产
木聚糖是在木质纤维素生物质的半纤维素部分中发现的一种重要多糖,可以水解为木糖,然后进一步脱水为糠醛(一种重要的可再生平台燃料前体)。在这里,结合分子模拟和实验证据,我们揭示了水-四氢呋喃(THF)助溶剂独特的温度依赖性相行为如何可以延迟木聚糖增溶作用,从而协同改善生物质向糠醛和5-HMF的催化共处理。我们的结果表明,基于聚合物构象行为与溶剂质量之间的聚合物相关性,助溶剂和水性环境均可用作木聚糖的“良好”溶剂。有趣的是,模拟还显示出与其他细胞壁成分不同(即,木质素和纤维素),木聚糖在THF-水中的溶剂化壳的组成取决于温度相的行为。在333 K和418 K之间的温度下,THF和水变得不溶混,THF从木聚糖的溶剂化壳中抽出,而高于和低于此温度范围,THF和水都存在于多糖的溶剂化壳中。这表明在333 K和418 K之间的温度下,木聚糖在THF-水中的增溶作用可能类似于纯水溶液,但在此范围之外有所不同。通过证明在可混溶温度范围内(445 K)在THF水中的木聚糖溶解度降低2倍,而在不混溶范围内(400 K)的溶解度不变,对沙滩木木聚糖的实验反应证实了这一假设。



























 京公网安备 11010802027423号
京公网安备 11010802027423号