当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
An Efficient Synthesis of exo/endo‐Hydroxylated Cyclohexenones by Thiol/Amine‐Mediated Tandem Aldol–[2,3]‐Sigmatropic Rearrangement: Amine‐Dependent Complementary Regioselectivity
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-04-06 , DOI: 10.1002/ajoc.201800081 Motofumi Miura 1 , Toshinori Nakakita 1, 2 , Masaharu Toriyama 1 , Shigeyasu Motohashi 1
Asian Journal of Organic Chemistry ( IF 2.7 ) Pub Date : 2018-04-06 , DOI: 10.1002/ajoc.201800081 Motofumi Miura 1 , Toshinori Nakakita 1, 2 , Masaharu Toriyama 1 , Shigeyasu Motohashi 1
Affiliation
|
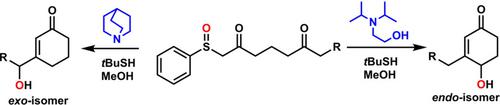
exo/endo‐Hydroxylated cyclohexenones are essential synthetic intermediates for a variety of biologically active compounds. However, methods that lead to efficient and selective preparation of exo/endo‐hydroxylated cyclohexenones remain to be established. Herein, we report the practical and scalable synthesis of exo/endo‐hydroxylated cyclohexenones from readily available 2,6‐diketosulfoxides. We demonstrate that endo and exo geometric isomers of hydroxylated cyclohexenones can be obtained by simple modification of the reaction conditions.
中文翻译:

硫醇/胺介导的串联Aldol– [2,3]-适马重排的高效合成外/内-羟基化环己烯酮:胺依赖的互补区域选择性
外/内羟基环己烯酮是多种生物活性化合物必不可少的合成中间体。然而,仍然需要建立能够有效,选择性地制备外/内羟基化环己烯酮的方法。在本文中,我们报道了从容易获得的2,6-二酮亚砜合成外切/内切-羟基化环己烯酮的实用且可扩展的方法。我们证明羟基化环己烯酮的内和外几何异构体可以通过简单修改反应条件来获得。
更新日期:2018-04-06
中文翻译:

硫醇/胺介导的串联Aldol– [2,3]-适马重排的高效合成外/内-羟基化环己烯酮:胺依赖的互补区域选择性
外/内羟基环己烯酮是多种生物活性化合物必不可少的合成中间体。然而,仍然需要建立能够有效,选择性地制备外/内羟基化环己烯酮的方法。在本文中,我们报道了从容易获得的2,6-二酮亚砜合成外切/内切-羟基化环己烯酮的实用且可扩展的方法。我们证明羟基化环己烯酮的内和外几何异构体可以通过简单修改反应条件来获得。


























 京公网安备 11010802027423号
京公网安备 11010802027423号