Journal of Inorganic Biochemistry ( IF 3.9 ) Pub Date : 2018-03-04 , DOI: 10.1016/j.jinorgbio.2018.03.001 Shaghayegh Dezvarei , Hiroki Onoda , Osami Shoji , Yoshihito Watanabe , Stephen G. Bell
|
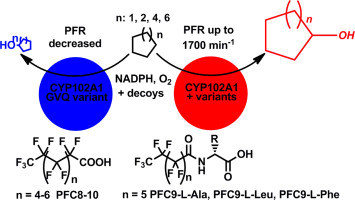
The wild-type cytochrome P450 (CYP) monooxygenase enzyme CYP102A1 (P450Bm3) has low activity for cycloalkane oxidation. The oxidation of these substrates by variants of this enzyme in combination with perfluorinated decoy molecules (PFCs) was investigated to improve productivity. The use of rate accelerating variants, which have mutations located outside of the substrate binding pocket as well as an active site variant of CYP102A1 (A74G/F87V/L188Q) all enhanced cycloalkane oxidation (C5 to C10). The addition of the decoy molecules to the wild-type and the rate accelerating mutants of CYP102A1 boosted the substrate oxidation rates even further. However, the levels of cycloalkanol product decreased with the larger alkanes when the decoy molecules were used with the variant A74G/F87V/L188Q, which contained mutations within the substrate binding pocket. For the majority of the enzymes and PFC decoy molecule combinations the highest levels of oxidation were obtained with cyclooctane. When larger second generation decoy molecules, based on modified amino acids were utilised there was a significant improvement in the oxidation of the smaller cycloalkanes by the wild-type enzyme and one other variant. This resulted in significant improvements in biocatalytic oxidation of cyclopentane and cyclohexane. However, the use of these optimised decoy molecules did not significantly improve cycloalkane oxidation over the fluorinated fatty acid derivatives when combined with the best rate accelerating variant, R47L/Y51F/I401P. Overall our approach enabled the cycloalkanes to be oxidised 300- to 8000-fold more efficiently than the wild-type enzyme at product formation rates in excess of 500 and up to 1700 nmol·nmol-CYP−1·min−1.
中文翻译:

通过将诱饵分子共添加到细胞色素P450 CYP102A1的变体中,有效地环化环烷烃
野生型细胞色素P450(CYP)单加氧酶CYP102A1(P450Bm3)对环烷烃的氧化活性较低。研究了这种酶的变体与全氟化诱饵分子(PFC)结合对这些底物的氧化作用,以提高生产率。使用具有位于底物结合口袋外部的突变的速率加速变体以及CYP102A1(A74G / F87V / L188Q)的活性位点变体,均可增强环烷氧化(C5至C10)。将诱饵分子添加到野生型和CYP102A1的加速突变体进一步提高了底物的氧化速率。但是,当诱饵分子与A74G / F87V / L188Q变体一起使用时,较大的烷烃会降低环烷醇产物的水平,其在底物结合口袋中包含突变。对于大多数酶和PFC诱饵分子组合,使用环辛烷可获得最高的氧化水平。当使用基于修饰氨基酸的较大的第二代诱饵分子时,野生型酶和另一种变体对较小的环烷烃的氧化作用得到了显着改善。这显着改善了环戊烷和环己烷的生物催化氧化。但是,当与最佳速率加速变体R47L / Y51F / I401P结合使用时,使用这些优化的诱饵分子不会比氟化脂肪酸衍生物显着改善环烷烃的氧化作用。-1 ·min -1。



























 京公网安备 11010802027423号
京公网安备 11010802027423号