当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Halogen bonds in 2,5-dihalopyridine-copper(ii) chloride complexes†
CrystEngComm ( IF 3.1 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8ce00209f Rakesh Puttreddy 1, 2, 3, 4 , Carolina von Essen 1, 2, 3, 4 , Anssi Peuronen 1, 2, 3, 4 , Manu Lahtinen 1, 2, 3, 4 , Kari Rissanen 1, 2, 3, 4
CrystEngComm ( IF 3.1 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1039/c8ce00209f Rakesh Puttreddy 1, 2, 3, 4 , Carolina von Essen 1, 2, 3, 4 , Anssi Peuronen 1, 2, 3, 4 , Manu Lahtinen 1, 2, 3, 4 , Kari Rissanen 1, 2, 3, 4
Affiliation
|
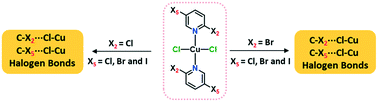
Ten coordination complexes obtained through a facile reaction between 2,5-dihalopyridines and copper(II) chloride (CuCl2) are characterized using single crystal X-ray diffraction. Two series of dihalopyridine complexes based on 2-chloro-5-X-pyridine and 2-bromo-5-X-pyridine (X = F, Cl, Br and I) were prepared to analyze the C–X2/X5⋯Cl–Cu halogen bonds (XB). The influence of X2- and X5-substituents on the respective interactions was examined by comparing them to the X2/X3⋯Cl–Cu XBs found in mono-substituted halopyridine complexes, (n-X-pyridine)2·CuCl2 (n = 2, 3 and X = Cl, Br and I). Varying the X5-halogens in (2,5-dihalopyridine)2·CuCl2, the C5–X5⋯Cl–Cu XBs follow the order F5 < Cl5 < Br5 < I5 for X2 = Cl and Cl5 < Br5 < I5 for X2 = Br. The C2–X2⋯Cl–Cu XB distances did not follow any particular trends, and are slightly longer compared to corresponding distances in (2-halopyridine)2·CuCl2 complexes due to the competition of X2 and X5-halogen based halogen bonds. The C3–X3⋯Cl–Cu contacts in (3-halopyridine)2·CuCl2 have RXB values > 1 and they cannot be considered as halogen bonds. This proves the polarization effect of X2- to X5- rather than X5- to X2-halogen, and the introduction of second halogen substituent in para-position to X5- triggers C2–X2⋯Cl–Cu and C5–X5⋯Cl–Cu XB interactions.
中文翻译:

2,5-二卤吡啶-铜(ii)氯化物络合物中的卤素键†
通过2,5-二卤代吡啶与氯化铜(II)(CuCl 2)的容易反应获得了十种配位络合物,使用单晶X射线衍射进行了表征。制备了两种基于2-氯-5-X-吡啶和2-溴-5-X-吡啶的二卤代吡啶配合物(X = F,Cl,Br和I)来分析C–X2 / X5⋯Cl–铜卤素键(XB)。通过将X2-和X5-取代基与单取代卤代吡啶络合物(n -X-吡啶)2 ·CuCl 2(n = 2,3和X = Cl,Br和I)。(2,5-二卤吡啶)2 ·CuCl 2中的X5-卤素变化,对于X2 = Cl,C5–X5sCl–Cu XB遵循以下顺序:F5 <Cl5 <Br5 <I5,对于X2 = Br,Cl5 <Br5 <I5。C2-X2⋯Cl-Cu XB的距离没有遵循任何特定的趋势,并且由于(基于X2和X5-卤素的卤素键的竞争)(2-卤代吡啶)2 ·CuCl 2络合物中的相应距离而略长。所述C3-X3⋯(3卤代吡啶)CL-铜触点2 ·的CuCl 2有- [R XB值> 1并且它们不能被认为是卤键。这证明了X2-X5-而不是X5- X2-卤素的极化作用,并且在X5-对位引入第二个卤素取代基会触发C2-X2XCl-Cu和C5-X5⋯Cl-Cu XB交互。
更新日期:2018-03-02
中文翻译:

2,5-二卤吡啶-铜(ii)氯化物络合物中的卤素键†
通过2,5-二卤代吡啶与氯化铜(II)(CuCl 2)的容易反应获得了十种配位络合物,使用单晶X射线衍射进行了表征。制备了两种基于2-氯-5-X-吡啶和2-溴-5-X-吡啶的二卤代吡啶配合物(X = F,Cl,Br和I)来分析C–X2 / X5⋯Cl–铜卤素键(XB)。通过将X2-和X5-取代基与单取代卤代吡啶络合物(n -X-吡啶)2 ·CuCl 2(n = 2,3和X = Cl,Br和I)。(2,5-二卤吡啶)2 ·CuCl 2中的X5-卤素变化,对于X2 = Cl,C5–X5sCl–Cu XB遵循以下顺序:F5 <Cl5 <Br5 <I5,对于X2 = Br,Cl5 <Br5 <I5。C2-X2⋯Cl-Cu XB的距离没有遵循任何特定的趋势,并且由于(基于X2和X5-卤素的卤素键的竞争)(2-卤代吡啶)2 ·CuCl 2络合物中的相应距离而略长。所述C3-X3⋯(3卤代吡啶)CL-铜触点2 ·的CuCl 2有- [R XB值> 1并且它们不能被认为是卤键。这证明了X2-X5-而不是X5- X2-卤素的极化作用,并且在X5-对位引入第二个卤素取代基会触发C2-X2XCl-Cu和C5-X5⋯Cl-Cu XB交互。



























 京公网安备 11010802027423号
京公网安备 11010802027423号