Acta Biomaterialia ( IF 9.7 ) Pub Date : 2018-03-05 , DOI: 10.1016/j.actbio.2018.02.032 V. Bonito , A.I.P.M. Smits , O.J.G.M. Goor , B.D. Ippel , A. Driessen-Mol , T.J.A.G. Münker , A.W. Bosman , T. Mes , P.Y.W. Dankers , C.V.C. Bouten
|
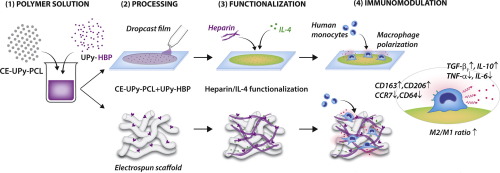
Hallmark of the in situ tissue engineering approach is the use of bioresorbable, synthetic, acellular scaffolds, which are designed to modulate the inflammatory response and actively trigger tissue regeneration by the body itself at the site of implantation. Much research is devoted to the design of synthetic materials modulating the polarization of macrophages, which are essential mediators of the early stages of the inflammatory response. Here, we present a novel method for the functionalization of elastomers based on synthetic peptide chemistry, supramolecular self-assembly, and immobilization of heparin and interleukin 4 (IL-4), which is known to skew the polarization of macrophages into the wound healing “M2” phenotype. Ureido-pyrimidinone (UPy)-modified chain extended polycaprolactone (CE-UPy-PCL) was mixed with a UPy-modified heparin binding peptide (UPy-HBP) to allow for immobilization of heparin, and further functionalization with IL-4 via its heparin binding domain. As a first proof of principle, CE-UPy-PCL and UPy-HBP were premixed in solution, dropcast and exposed to primary human monocyte-derived macrophages, in the presence or absence of IL-4-heparin functionalization. It was demonstrated that the supramolecular IL-4-heparin functionalization effectively promoted macrophage polarization into an anti-inflammatory phenotype, in terms of morphology, immunohistochemistry and cytokine secretion. Moreover, the supramolecular functionalization approach used was successfully translated to 3D electrospun scaffolds for in situ tissue engineering purposes, where UPy-HBP retention, and heparin and IL-4 attachment to the supramolecular scaffolds were proven over 7 days. Lastly, human monocyte-derived macrophages were cultured on functionalized 3D scaffolds and analyzed in terms of protein secretion. This study presents a novel method in designing a versatile class of functionalized elastomers that effectively harness the anti-inflammatory behavior of macrophages in vitro, and as such, may be instrumental for the development of a new class of synthetic materials for in situ tissue engineering purposes.
Statement of significance
Macrophages and their phenotypic and functional plasticity play a pivotal role in metabolic homeostasis and tissue repair. Based on this notion, bioactivated materials modulating macrophage polarization were extensively investigated in the past. Here, we designed immunomodulating, synthetic materials based on supramolecular immobilization of a heparin binding peptide, and further bioactivation with heparin and IL-4, an anti-inflammatory cytokine responsible for M2 activation and polarization. Human monocyte-derived macrophages cultured on heparin-IL-4 biactivated materials displayed an elongated morphology and an anti-inflammatory phenotype, with downregulation of pro-inflammatory cytokines and promotion of anti-inflammatory cytokines over time. This study represents the first step in designing a novel class of synthetic, bioactivated materials that harness the regenerative behavior of host macrophages towards in situ tissue regeneration.
中文翻译:

通过肝素-IL-4功能化的超分子弹性体调节巨噬细胞表型和蛋白质分泌
原位标记组织工程学方法是使用可生物吸收的,合成的,无细胞的支架,这些支架旨在调节炎症反应并在植入部位由人体自身主动触发组织再生。大量研究致力于调节巨噬细胞极化的合成材料的设计,巨噬细胞是炎症反应早期的重要介质。在这里,我们介绍了一种基于合成肽化学,超分子自组装以及肝素和白介素4(IL-4)固定化的弹性体功能化的新方法,已知该方法会使巨噬细胞的极化偏向伤口愈合“ M2”表型。将Ureido-嘧啶酮(UPy)修饰的扩链聚己内酯(CE-UPy-PCL)与UPy修饰的肝素结合肽(UPy-HBP)混合以固定肝素,并通过其肝素进一步用IL-4进行功能化结合域。作为原理的第一个证明,在存在或不存在IL-4-肝素功能化的情况下,将CE-UPy-PCL和UPy-HBP在溶液中预混合,滴铸并暴露于原代人单核细胞衍生的巨噬细胞。从形态,免疫组织化学和细胞因子分泌方面,证明了超分子IL-4-肝素功能化有效地促进巨噬细胞极化为抗炎表型。此外,所使用的超分子功能化方法已成功翻译为3D电纺支架,用于 并通过其肝素结合结构域进一步用IL-4进行功能化。作为原理的第一个证明,在存在或不存在IL-4-肝素功能化的情况下,将CE-UPy-PCL和UPy-HBP在溶液中预混合,滴铸并暴露于原代人单核细胞衍生的巨噬细胞。从形态,免疫组织化学和细胞因子分泌方面,证明了超分子IL-4-肝素功能化有效地促进巨噬细胞极化为抗炎表型。此外,所使用的超分子功能化方法已成功翻译为3D电纺支架,用于 并通过其肝素结合结构域进一步用IL-4进行功能化。作为原理的第一个证明,在存在或不存在IL-4-肝素功能化的情况下,将CE-UPy-PCL和UPy-HBP在溶液中预混合,滴铸并暴露于原代人单核细胞衍生的巨噬细胞。从形态,免疫组织化学和细胞因子分泌方面,证明了超分子IL-4-肝素功能化有效地促进巨噬细胞极化为抗炎表型。此外,所使用的超分子功能化方法已成功翻译为3D电纺支架,用于 在存在或不存在IL-4-肝素功能化的情况下。从形态,免疫组织化学和细胞因子分泌方面,证明了超分子IL-4-肝素功能化有效地促进巨噬细胞极化为抗炎表型。此外,所使用的超分子功能化方法已成功翻译为3D电纺支架,用于 在存在或不存在IL-4-肝素功能化的情况下。从形态,免疫组织化学和细胞因子分泌方面,证明了超分子IL-4-肝素功能化有效地促进巨噬细胞极化为抗炎表型。此外,所使用的超分子功能化方法已成功翻译为3D电纺支架,用于原位组织工程目的,其中UPy-HBP保留,肝素和IL-4与超分子支架的附着在7天之内得到了证明。最后,将人类单核细胞衍生的巨噬细胞培养在功能化的3D支架上,并根据蛋白质分泌进行分析。这项研究提出了一种设计通用型功能化弹性体的新颖方法,该类弹性体可有效利用体外巨噬细胞的抗炎行为,因此,可能有助于开发用于原位组织工程目的的新型合成材料。
重要声明
巨噬细胞及其表型和功能可塑性在代谢稳态和组织修复中发挥关键作用。基于该概念,过去已经广泛地研究了调节巨噬细胞极化的生物活化材料。在这里,我们基于肝素结合肽的超分子固定,并进一步利用肝素和IL-4(负责M2激活和极化的抗炎细胞因子)进行生物激活,设计了免疫调节合成材料。在肝素-IL-4双活化材料上培养的人单核细胞衍生巨噬细胞显示出细长的形态和抗炎表型,随着时间的流逝,促炎细胞因子的表达下调并促进了消炎细胞因子的表达。这项研究代表了设计新颖类别的合成生物活化材料的第一步,该材料利用了宿主巨噬细胞向原位的再生行为 组织再生。



























 京公网安备 11010802027423号
京公网安备 11010802027423号