当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
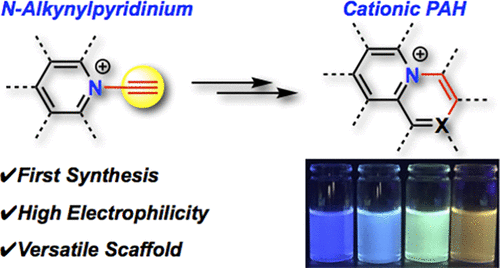
N-Alkynylpyridinium Salts: Highly Electrophilic Alkyne-Pyridine Conjugates as Precursors of Cationic Nitrogen-Embedded Polycyclic Aromatic Hydrocarbons
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-05 , DOI: 10.1021/jacs.8b00356 Naoyuki Toriumi 1 , Norihito Asano 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Masanobu Uchiyama 1, 2
Journal of the American Chemical Society ( IF 15.0 ) Pub Date : 2018-03-05 , DOI: 10.1021/jacs.8b00356 Naoyuki Toriumi 1 , Norihito Asano 1 , Kazunori Miyamoto 1 , Atsuya Muranaka 2 , Masanobu Uchiyama 1, 2
Affiliation
|
We achieved the first synthesis of N-alkynylpyridinium salts, by reacting pyridines with alkynyl-λ3-iodanes. The N-alkynylpyridiniums exhibit highly electron-accepting character with extended π-conjugation. The electrophilic alkynyl groups were readily susceptible to Michael addition and 1,3-dipolar cycloaddition to afford various N-alkenylpyridiniums. Ring-fused pyridiniums were synthesized through intramolecular cyclization, demonstrating the utility of N-alkynylpyridiniums for the design of various electron-deficient cationic nitrogen-embedded polycyclic aromatic hydrocarbons with unique optical and electrochemical properties.
中文翻译:

N-炔基吡啶鎓盐:高亲电性炔-吡啶共轭物作为阳离子氮嵌入多环芳烃的前体
我们通过吡啶与炔基-λ3-碘反应,首次合成了 N-炔基吡啶鎓盐。N-炔基吡啶鎓表现出高度的电子接受特性,并具有扩展的 π 共轭。亲电炔基很容易受到迈克尔加成和 1,3-偶极环加成的影响,得到各种 N-烯基吡啶鎓。稠环吡啶鎓是通过分子内环化合成的,证明了 N-炔基吡啶鎓可用于设计具有独特光学和电化学性质的各种缺电子阳离子氮嵌入多环芳烃。
更新日期:2018-03-05
中文翻译:

N-炔基吡啶鎓盐:高亲电性炔-吡啶共轭物作为阳离子氮嵌入多环芳烃的前体
我们通过吡啶与炔基-λ3-碘反应,首次合成了 N-炔基吡啶鎓盐。N-炔基吡啶鎓表现出高度的电子接受特性,并具有扩展的 π 共轭。亲电炔基很容易受到迈克尔加成和 1,3-偶极环加成的影响,得到各种 N-烯基吡啶鎓。稠环吡啶鎓是通过分子内环化合成的,证明了 N-炔基吡啶鎓可用于设计具有独特光学和电化学性质的各种缺电子阳离子氮嵌入多环芳烃。



























 京公网安备 11010802027423号
京公网安备 11010802027423号