当前位置:
X-MOL 学术
›
J. Mater. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Hierarchical theranostic nanomedicine: MRI contrast agents as a physical vehicle anchor for high drug loading and triggered on-demand delivery†
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8tb00135a Guangqin Liu 1, 2, 3, 4, 5 , Jian Deng 1, 2, 3, 4, 5 , Fang Liu 1, 2, 3, 4, 5 , Zheng Wang 1, 2, 3, 4, 5 , Dan Peer 6, 7, 8, 9, 10 , Yanjun Zhao 1, 2, 3, 4, 5
Journal of Materials Chemistry B ( IF 7 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8tb00135a Guangqin Liu 1, 2, 3, 4, 5 , Jian Deng 1, 2, 3, 4, 5 , Fang Liu 1, 2, 3, 4, 5 , Zheng Wang 1, 2, 3, 4, 5 , Dan Peer 6, 7, 8, 9, 10 , Yanjun Zhao 1, 2, 3, 4, 5
Affiliation
|
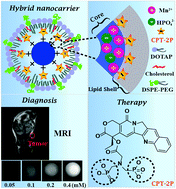
The loading of drugs and imaging agents in theranostic nanomedicines by a physical approach is usually poor (<5%), which limits their therapeutic effect and translation potential. We report a hierachical hybrid nanocarrier made of a manganese phosphate core, an outermost lipid shell, and an electrostatically deposited campothecin phosphonate interfacial middle layer. The hydrophobic interactions between camptothecin and lipid layers maintained the integrity and stability of the hybrid nanocarrier. Such a nanoplatform could surprisingly load camptothecin over 15% (w/w) with decent serum stability. The nanocarrier displayed pH-dependent cargo release profiles due to particle collapse under acidic conditions under which the r1 relaxivity of magnetic resonance imaging (MRI) was 25.2 mM−1 s−1 (pH 5.0). The nanocarrier could efficiently transport camptothecin into 4T1 cells with a half maximal inhibitory concentration of 5.4 ± 0.3 μM. Both in vivo MRI and fluorescence imaging analysis revealed that the nanocarrier could competently deliver the cargo to the tumor site. The anticancer efficacy of camptothecin-loaded nanocarrier was proved using the same 4T1 tumor-bearing mice model coupled with the histological and apoptosis analysis. This work not only presented a novel drug encapsulation approach, but also provided a new theranostic hybrid nanoplatform which could realize MRI-guided delivery of hydrophobic agents.
中文翻译:

层级治疗性纳米医学:MRI造影剂作为高载药量和触发按需递送的物理媒介锚†
通过物理方法在治疗性纳米药物中药物和显像剂的负载通常较差(<5%),这限制了它们的治疗效果和翻译潜力。我们报告了由磷酸锰核,最外层脂质壳和静电沉积的喜树碱膦酸酯界面中间层组成的层级混合纳米载体。喜树碱和脂质层之间的疏水相互作用保持了杂化纳米载体的完整性和稳定性。这样的纳米平台可以令人惊讶地将喜树碱负载超过15%(w / w),并具有良好的血清稳定性。由于在酸性条件下的颗粒塌陷,纳米载体显示出pH依赖的货物释放曲线,在酸性条件下,磁共振成像(MRI)的r 1弛豫度为25.2 mM -1s -1(pH 5.0)。纳米载体可以将喜树碱有效地转运到4T1细胞中,最大抑制浓度为5.4±0.3μM。无论在体内MRI和荧光成像分析表明,纳米载体可胜任交付货物到肿瘤部位。使用相同的4T1荷瘤小鼠模型,结合组织学和凋亡分析,证明了喜树碱纳米载体的抗癌作用。这项工作不仅提出了一种新颖的药物包封方法,而且还提供了一种新型的治疗学杂合纳米平台,可以实现MRI指导的疏水剂的递送。
更新日期:2018-03-05
中文翻译:

层级治疗性纳米医学:MRI造影剂作为高载药量和触发按需递送的物理媒介锚†
通过物理方法在治疗性纳米药物中药物和显像剂的负载通常较差(<5%),这限制了它们的治疗效果和翻译潜力。我们报告了由磷酸锰核,最外层脂质壳和静电沉积的喜树碱膦酸酯界面中间层组成的层级混合纳米载体。喜树碱和脂质层之间的疏水相互作用保持了杂化纳米载体的完整性和稳定性。这样的纳米平台可以令人惊讶地将喜树碱负载超过15%(w / w),并具有良好的血清稳定性。由于在酸性条件下的颗粒塌陷,纳米载体显示出pH依赖的货物释放曲线,在酸性条件下,磁共振成像(MRI)的r 1弛豫度为25.2 mM -1s -1(pH 5.0)。纳米载体可以将喜树碱有效地转运到4T1细胞中,最大抑制浓度为5.4±0.3μM。无论在体内MRI和荧光成像分析表明,纳米载体可胜任交付货物到肿瘤部位。使用相同的4T1荷瘤小鼠模型,结合组织学和凋亡分析,证明了喜树碱纳米载体的抗癌作用。这项工作不仅提出了一种新颖的药物包封方法,而且还提供了一种新型的治疗学杂合纳米平台,可以实现MRI指导的疏水剂的递送。

























 京公网安备 11010802027423号
京公网安备 11010802027423号