当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
A novel symmetrically multifunctionalized dodecamethoxy-cycloparaphenylene: synthesis, photophysical, and supramolecular properties†
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8qo00033f Dapeng Lu 1, 2, 3, 4, 5 , Guilin Zhuang 6, 7, 8, 9 , Hongxing Jia 1, 2, 3, 4, 5 , Jinyi Wang 1, 2, 3, 4, 5 , Qiang Huang 1, 2, 3, 4, 5 , Shengsheng Cui 1, 2, 3, 4, 5 , Pingwu Du 1, 2, 3, 4, 5
Organic Chemistry Frontiers ( IF 5.4 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c8qo00033f Dapeng Lu 1, 2, 3, 4, 5 , Guilin Zhuang 6, 7, 8, 9 , Hongxing Jia 1, 2, 3, 4, 5 , Jinyi Wang 1, 2, 3, 4, 5 , Qiang Huang 1, 2, 3, 4, 5 , Shengsheng Cui 1, 2, 3, 4, 5 , Pingwu Du 1, 2, 3, 4, 5
Affiliation
|
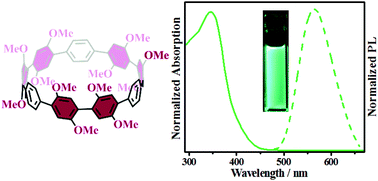
In the present study, we report the synthesis of a novel symmetrically multifunctionalized cycloparaphenylene (CPP), dodecamethoxy-[9]CPP (ring[9]arene), through nickel-mediated macrocyclization and subsequent reductive aromatization reactions. The electron-donating methoxy groups were introduced onto the curved precursor at an early stage to avoid the problem of non-selective functionalization of CPPs. Compared to [9]CPP, the present dodecamethoxy-[9]CPP shows a significant red shift (>73 nm) in the fluorescence spectrum. In addition, theoretical calculations revealed its increased torsion angles and ring strain (71.01 kcal mol−1) compared with its unsubstituted counterpart.
中文翻译:

一种新型的对称多官能化的十二甲氧基-环对亚苯基:合成,光物理和超分子性质†
在本研究中,我们报道了通过镍介导的大环化和随后的还原性芳构化反应合成的新型对称多官能环对亚苯基(CPP),十二甲氧基-[9] CPP(环[9]芳烃)。给电子的甲氧基基团在早期被引入到弯曲的前体上,以避免CPP的非选择性官能化的问题。与[9] CPP相比,目前的十二甲氧基-[9] CPP在荧光光谱中显示出明显的红移(> 73 nm)。此外,理论计算表明,与未取代的同类产品相比,其扭转角和环应变(71.01 kcal mol -1)有所增加。
更新日期:2018-03-05
中文翻译:

一种新型的对称多官能化的十二甲氧基-环对亚苯基:合成,光物理和超分子性质†
在本研究中,我们报道了通过镍介导的大环化和随后的还原性芳构化反应合成的新型对称多官能环对亚苯基(CPP),十二甲氧基-[9] CPP(环[9]芳烃)。给电子的甲氧基基团在早期被引入到弯曲的前体上,以避免CPP的非选择性官能化的问题。与[9] CPP相比,目前的十二甲氧基-[9] CPP在荧光光谱中显示出明显的红移(> 73 nm)。此外,理论计算表明,与未取代的同类产品相比,其扭转角和环应变(71.01 kcal mol -1)有所增加。



























 京公网安备 11010802027423号
京公网安备 11010802027423号