当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Li deposition and desolvation with electron transfer at a silicon/propylene-carbonate interface: transition-state and free-energy profiles by large-scale first-principles molecular dynamics†
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c7cp08569a Tsukuru Ohwaki 1, 2, 3, 4, 5 , Taisuke Ozaki 5, 6, 7, 8 , Yukihiro Okuno 5, 9, 10, 11 , Tamio Ikeshoji 1, 2, 3, 4, 5 , Hideto Imai 1, 2, 3, 4, 5 , Minoru Otani 5, 12, 13, 14
Physical Chemistry Chemical Physics ( IF 3.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1039/c7cp08569a Tsukuru Ohwaki 1, 2, 3, 4, 5 , Taisuke Ozaki 5, 6, 7, 8 , Yukihiro Okuno 5, 9, 10, 11 , Tamio Ikeshoji 1, 2, 3, 4, 5 , Hideto Imai 1, 2, 3, 4, 5 , Minoru Otani 5, 12, 13, 14
Affiliation
|
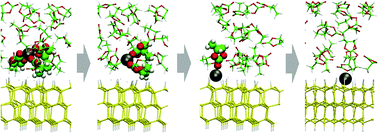
We report the result of a large-scale first-principles molecular dynamics simulation under different electric biases performed to understand the charge transfer process coupling with lithium deposition and desolvation processes. We applied the effective screening medium (ESM) method to control the bias across the electrode/solution interface, and simulated a series of Li de-solvation and Li-deposition reactions occurring under the bias. Solvated Li+ in the bulk propylene carbonate migrates to the Si electrode surface and gradually de-solvates through the transition state. Introducing the blue-moon ensemble method, we determined the possible structures and activation energies for the transition states.
中文翻译:

锂在硅/丙烯-碳酸酯界面上的沉积和通过电子转移的去溶剂化:通过大规模第一性原理分子动力学获得的过渡态和自由能曲线†
我们报告了在不同的电偏压下进行的大规模第一性原理分子动力学模拟的结果,以了解与锂沉积和去溶剂化过程耦合的电荷转移过程。我们应用有效的筛选介质(ESM)方法来控制电极/溶液界面上的偏压,并模拟了在该偏压下发生的一系列Li脱溶剂和Li沉积反应。本体碳酸亚丙酯中的溶剂化Li +迁移至Si电极表面,并通过过渡态逐渐脱溶剂。引入蓝月合奏法,我们确定了过渡态的可能结构和活化能。
更新日期:2018-03-05
中文翻译:

锂在硅/丙烯-碳酸酯界面上的沉积和通过电子转移的去溶剂化:通过大规模第一性原理分子动力学获得的过渡态和自由能曲线†
我们报告了在不同的电偏压下进行的大规模第一性原理分子动力学模拟的结果,以了解与锂沉积和去溶剂化过程耦合的电荷转移过程。我们应用有效的筛选介质(ESM)方法来控制电极/溶液界面上的偏压,并模拟了在该偏压下发生的一系列Li脱溶剂和Li沉积反应。本体碳酸亚丙酯中的溶剂化Li +迁移至Si电极表面,并通过过渡态逐渐脱溶剂。引入蓝月合奏法,我们确定了过渡态的可能结构和活化能。


























 京公网安备 11010802027423号
京公网安备 11010802027423号