当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Selectivity in Hydrogenation Catalysis with Unsaturated Aldehydes: Parallel versus Sequential Steps
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jpclett.8b00173 Yujung Dong 1 , Francisco Zaera 1
The Journal of Physical Chemistry Letters ( IF 5.7 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.jpclett.8b00173 Yujung Dong 1 , Francisco Zaera 1
Affiliation
|
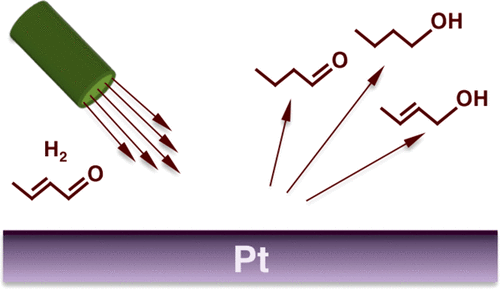
A high-flux molecular beam setup has been used to characterize the kinetics of the steady-state catalytic hydrogenation of unsaturated aldehydes, specifically of crotonaldehyde, promoted by platinum surfaces under single-collision conditions. Surprisingly, in addition to the hydrogenation of the individual single bonds, to yield the saturated aldehyde and the unsaturated alcohol, the formation of the saturated alcohol, the product of the hydrogenation of both C═C and C═O bonds, was detected as well. This indicates that the dual hydrogenation reaction is a primary pathway and not the result of secondary hydrogenation of the other products as commonly assumed. Moreover, an increase in the partial pressure of the reactant was found to shift the reaction selectivity from the saturated alcohol to the saturated aldehyde without significantly affecting the selectivity toward the production of the unsaturated alcohol. We explain these observations by proposing a mechanism involving the parallel formation of several monohydrogenated intermediates on the surface.
中文翻译:

不饱和醛加氢催化的选择性:平行步骤与顺序步骤
高通量分子束装置已用于表征不饱和醛(特别是巴豆醛)的稳态催化氢化动力学,该动力学由铂在单碰撞条件下促进。令人惊讶地,除了单独的单键的氢化以外,为了产生饱和醛和不饱和醇,还检测到饱和醇的形成,即C═C和C═O键的氢化产物。 。这表明双重氢化反应是主要途径,而不是通常假定的其他产物二次氢化的结果。而且,发现反应物分压的增加使反应选择性从饱和醇转变为饱和醛,而没有显着影响向不饱和醇生产的选择性。我们通过提出一种涉及在表面上平行形成几种单氢化中间体的机理来解释这些发现。
更新日期:2018-03-02
中文翻译:

不饱和醛加氢催化的选择性:平行步骤与顺序步骤
高通量分子束装置已用于表征不饱和醛(特别是巴豆醛)的稳态催化氢化动力学,该动力学由铂在单碰撞条件下促进。令人惊讶地,除了单独的单键的氢化以外,为了产生饱和醛和不饱和醇,还检测到饱和醇的形成,即C═C和C═O键的氢化产物。 。这表明双重氢化反应是主要途径,而不是通常假定的其他产物二次氢化的结果。而且,发现反应物分压的增加使反应选择性从饱和醇转变为饱和醛,而没有显着影响向不饱和醇生产的选择性。我们通过提出一种涉及在表面上平行形成几种单氢化中间体的机理来解释这些发现。



























 京公网安备 11010802027423号
京公网安备 11010802027423号