当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Ruthenium(II)-Catalyzed Redox-Free [3 + 2] Cycloaddition of N-Sulfonyl Aromatic Aldimines with Maleimides
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.joc.8b00102 Masilamani Tamizmani 1 , Balu Ramesh 1 , Masilamani Jeganmohan 1
The Journal of Organic Chemistry ( IF 3.6 ) Pub Date : 2018-03-02 00:00:00 , DOI: 10.1021/acs.joc.8b00102 Masilamani Tamizmani 1 , Balu Ramesh 1 , Masilamani Jeganmohan 1
Affiliation
|
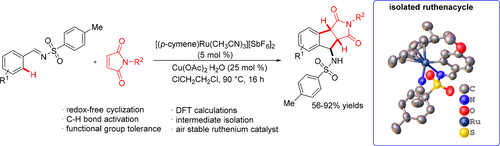
A ruthenium(II)-catalyzed redox-free cycloaddition of N-sulfonyl aromatic aldimines with maleimides providing 1-aminoindanes in good yields is described. Usually, maleimides reacted with substituted aromatics, affording the Michael-type ortho alkylated aromatics or 1,1-type cyclized spirosuccinimides. In the present reaction, maleimides provided 1,2-type cycloaddition products. The proposed mechanism was strongly supported by the DFT calculations and isolation of a ruthenacycle intermediate.
中文翻译:

钌(II)催化的N-磺酰基芳香醛亚胺与马来酰亚胺的无氧化还原[3 + 2]环加成反应
描述了钌(II)催化的N-磺酰基芳族醛亚胺与马来酰亚胺的无氧化还原的环加成,其以良好的产率提供了1-氨基茚满。通常,马来酰亚胺与取代的芳族化合物反应,得到迈克尔型邻烷基化的芳族化合物或1,1-型环化螺琥珀酰亚胺。在本反应中,马来酰亚胺提供了1,2-型环加成产物。拟议的机制得到DFT计算和钌环中间体中间体的分离的有力支持。
更新日期:2018-03-02
中文翻译:

钌(II)催化的N-磺酰基芳香醛亚胺与马来酰亚胺的无氧化还原[3 + 2]环加成反应
描述了钌(II)催化的N-磺酰基芳族醛亚胺与马来酰亚胺的无氧化还原的环加成,其以良好的产率提供了1-氨基茚满。通常,马来酰亚胺与取代的芳族化合物反应,得到迈克尔型邻烷基化的芳族化合物或1,1-型环化螺琥珀酰亚胺。在本反应中,马来酰亚胺提供了1,2-型环加成产物。拟议的机制得到DFT计算和钌环中间体中间体的分离的有力支持。


























 京公网安备 11010802027423号
京公网安备 11010802027423号