Journal of Fluorine Chemistry ( IF 1.9 ) Pub Date : 2018-03-01 , DOI: 10.1016/j.jfluchem.2018.02.012 Boris I. Usachev
|
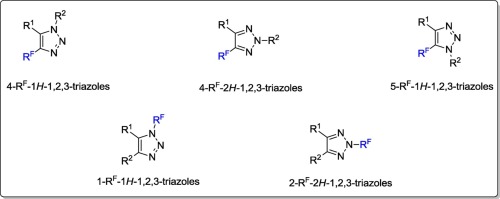
This review is devoted to the chemistry of fluoroalkyl-substituted 1,2,3-triazoles: their synthesis and chemical properties. Synthesis of C-fluoroalkyl-substituted 1,2,3-triazoles and N-fluoroalkyl-substituted 1,2,3-triazoles is considered. C-Fluoroalkyl-substituted 1,2,3-triazoles can be prepared via 1,3-dipolar cycloaddition, cyclizations of RF-vinyl azides, deoxyfluorination of alcohols, transformation of CO into CF2H, transformation of CO2H into CF3, electrophilic α-fluorination of 4/5-alkyl-1,2,3-triazoles, and using other methods. N-Fluoroalkyl-substituted 1,2,3-triazoles can be prepared via N-difluoromethylation of 1,2,3-triazoles, their N-trifluoromethylation with Togni’s reagent, N-fluoroalkylation with haloethylenes, using 1,3-dipolar cycloaddition and other methods. Fluoroalkyl-substituted 1,2,3-triazoles are chemically reactive substances, and they can be used as building-blocks for the preparation of medicinally and biologically beneficial compounds.
中文翻译:

氟烷基取代的1,2,3-三唑的化学
这篇评论致力于氟烷基取代的1,2,3-三唑的化学性质:它们的合成和化学性质。考虑了C-氟代烷基取代的1,2,3-三唑和N-氟代烷基取代的1,2,3-三唑的合成。可以通过1,3-偶极环加成,R F-乙烯基叠氮化物的环化,醇的脱氧氟化,CO转化为CF 2 H,CO 2 H转化为CF来制备C-氟烷基取代的1,2,3-三唑。参照图3,使用其他方法对4 / 5-烷基-1,2,3-三唑进行亲电α-氟化。N-氟烷基取代的1,2,3-三唑可通过N制备1,2,3-三唑的-二氟甲基化,用Togni's试剂的N-三氟甲基化,卤代乙烯的N-氟烷基化,使用1,3-偶极环加成法和其他方法。氟烷基取代的1,2,3-三唑是化学反应性物质,它们可用作制备具有医学和生物学意义的化合物的基础材料。



























 京公网安备 11010802027423号
京公网安备 11010802027423号